 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > Biological Functions and Clinical Applications of Distinct CD3 Subunits 
CD3, including subunits: CD3ε, CD3δ, CD3γ, and CD3ζ, serves as the central component of the TCR-CD3 complex, pivotal for T cell recognition and response to antigens. Each subunit of CD3 possesses unique biological functions and finds extensive clinical applications. For instance, CD3ε plays a crucial role in bispecific antibodies and immunotherapy, CD3δ exhibits promise in treating immune deficiencies, and CD3ζ is indispensable in CAR-T therapy. A thorough exploration of the functions and applications of CD3 subunits is poised to advance immunotherapy and offer novel approaches for treating cancer and immune-related disorders.
T cell receptor (TCR) categorized mainly into TCRαβ and TCRγδ. In humans, TCRαβ predominates and exhibits highly specific recognition of antigens presented by the Major Histocompatibility Complex (MHC). However, mature TCRαβ dimer lacks signaling domains to independently activate T cells. Therefore, it associates closely with CD3 molecules to form the TCR-CD3 complex, which facilitates signal transduction through synergistic interactions.
The 4 subunits of CD3 complex binding to TCRαβ in a precise stoichiometric ratio (TCRαβ: CD3γε: CD3δε: CD3ζζ = 1:1:1:1) to ensure complex stability and optimize signal transmission accuracy and efficiency.
Upon antigen stimulation, conformational changes occur in the intracellular domains of CD3. Src family protein tyrosine kinases (PTKs) phosphorylate tyrosine residues within the immune receptor tyrosine activation motif (ITAM) of the TCR/CD3 complex, creating docking sites for proteins with SH2 domains. Zeta-chain-associated protein kinase (ZAP-70), recruited by phosphorylated ITAMs, subsequently initiates downstream signaling pathways.
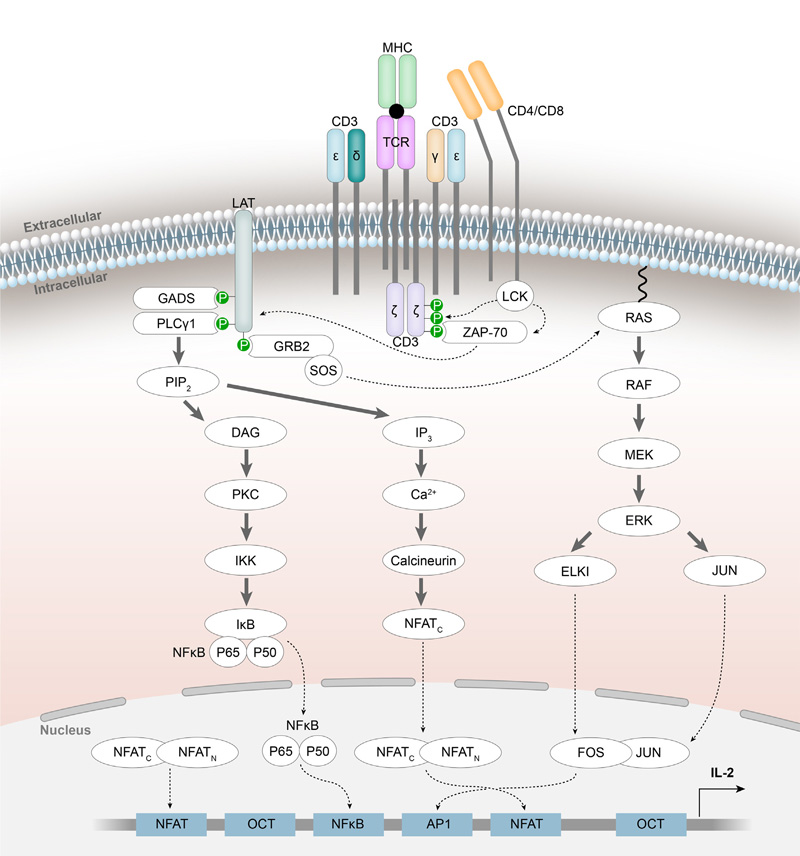
https://doi.org/10.1016/j.drudis.2022.04.019
Signal pathways related to TCR/CD3 complex
Each subunit of CD3 has a unique biological function, playing an indispensable role in T cell development, activation, signal transduction, and immune regulation through synergistic interactions.
CD3ε, as a signaling center, is crucial in the adaptive immune response. It not only participates in the TCR signaling pathway but also affects the positive and negative selection of thymic T cells, regulates cell surface receptor signaling pathways, and promotes T cell differentiation and activation. Additionally, CD3ε is involved in cerebellar development, synaptic growth, and the regulation of various signaling pathways, such as the transmembrane receptor protein tyrosine kinase signaling pathway and the apoptosis signaling pathway, significantly impacting gene expression and apoptosis processes.
CD3γ contributes to the establishment and maintenance of cell polarity, the complex assembly and transport of intracellular proteins, and plays a role in lymphocyte apoptosis. Although CD3δ overlaps functionally with CD3ε and CD3γ, co-regulating TCR signaling, its unique biological functions require further study. CD3ζ is involved in T cell activation, promotes IL-2 production, positively regulates protein localization, and plays a role in T cell antiviral defense.
• CD3ε
Given the pivotal role of CD3ε in T cell activation, the use of bispecific antibodies targeting CD3ε and tumor-associated antigens (TAA) for anti-tumor therapy has become a significant area of research. These bispecific antibodies can narrow the distance between tumor cells and effector T cells, and effectively kill tumor cells. For instance, Amgen's Blinatumomab is a bispecific antibody targeting CD3ε and CD19, used to treat B-cell acute lymphoblastic leukemia (B-ALL). The drug achieves its therapeutic goals by simultaneously binding to CD3ε on T cells and CD19 on tumor cells, thereby redirecting T cells to the tumor cells and triggering T-cell-mediated cytotoxicity.
Moreover, CD3ε is frequently used as a crucial research tool in the development of immunotherapy drugs. For example, humanized mouse models constructed with CD3ε can simulate T cell activation in humans, providing an essential platform for evaluating the efficacy and safety of drugs targeting CD3ε. Additionally, CD3ε can serve as a target for drug screening and optimization, aiding researchers in identifying immunotherapy drugs with better efficacy and lower toxicity. It is worth noting that the binding epitope and cd3ε binding conformation of CD3ε-targeting bispecific antibodies requires further investigation, as the known bispecific antibodies exhibit different binding characteristics for CD3 molecules.
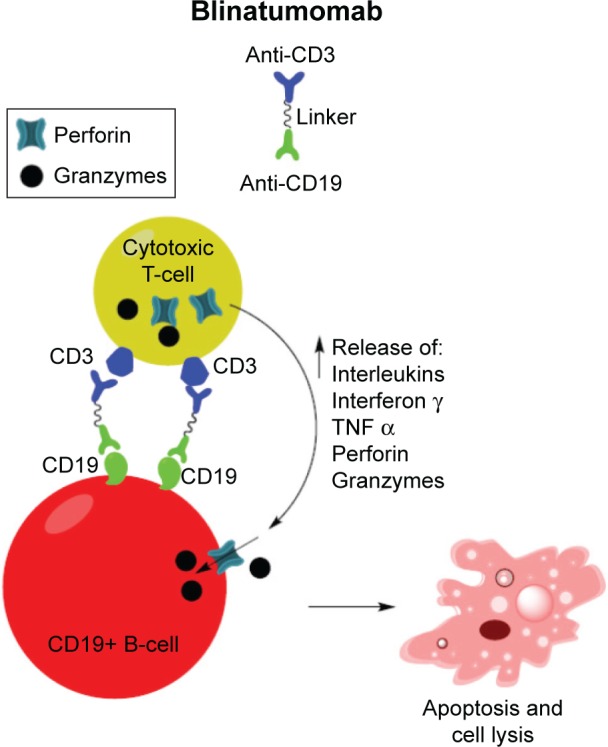
https://doi.org/10.2147/TCRM.S84261
Blinatumomab structure and mechanism of action
• CD3δ
While the clinical applications of CD3δ as a single target has not garnered as much attention as the CD3 complex or the combination of CD3 with other targets, recent studies have highlighted the critical role of CD3δ in CD3δ-severe combined immune deficiency (CD3δ-SCID). This disease is caused by a single-base mutation in the CD3δ gene, leading to early termination of the CD3δ protein translation. The CD3δ protein is essential for the normal development of blood stem cells into T cells. Its loss or abnormal function severely impacts T cell production and function, leaving patients with almost no immunity and facing serious life-threatening conditions.
Scientists are exploring ways to correct mutations in the CD3δ gene using gene editing techniques such as CRISPR-Cas9 and base editing, with the hope of restoring immune function in affected patients. Additionally, in-depth studies of CD3δ function and its interactions with other CD3 subunits provide new insights into the molecular mechanisms of T cell development and immune regulation. These studies also offer a theoretical foundation for developing therapies against other immune-related diseases.
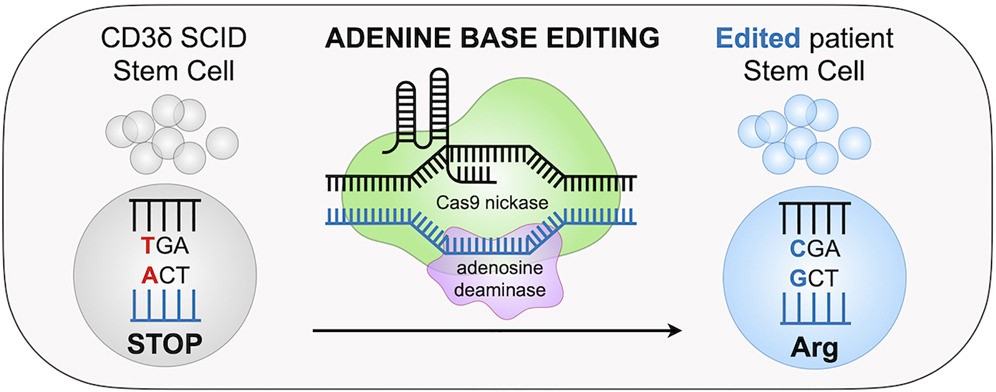
https://doi.org/10.1016/j.cell.2023.02.027
Adenine base editor (ABE) was used to catalyze the A·T→G·C conversion to repair disease-causing mutations in CD3δ-SCID patients
• CD3γ
The clinical applications of CD3γ primarily relates to its crucial role in T cell activation and signal transduction. However, direct clinical applications of CD3γ are relatively rare. As research on the mechanisms of T cell immune responses progresses and new immunotherapies are developed, CD3γ may become more significant in clinical treatments.
• CD3ζ
The intracellular domain of CD3ζ contains three ITAMs, which are essential for TCR signaling. In CAR-T cells, the intracellular domain of CAR binds to CD3ζ, enhancing the T cells' ability to recognize and kill tumor cells. FDA-approved CAR-T products such as Tisagenlecleucel and Axicabtagene Ciloleucel use CD3ζ as a signal transduction domain to achieve specific recognition and killing of tumor cells. This design has been widely adopted in CAR-T therapy, resulting in remarkable clinical outcomes.
TCR-CD3 complex is crucial in the recognition and response of T cells to antigens, and each CD3 subunit plays a unique role in T cell activation, signaling and immune regulation, and has important clinical applications. By gaining a deeper understanding of the structure and function of the CD3 complex, more effective immunotherapy strategies can be developed, offering new hope for the treatment of cancer and immune-related diseases.
Due to the unique physiological characteristics, CD3 molecules, especially highly homogeneous heterodimers, are particularly important. Selecting the appropriate product from our extensive range of CD3 molecules will meet your needs for exploring CD3's biological functions and clinical applications.
| CD3E & CD3D | CD3E & CD3G | CD3 epsilon | CD3 delta | CD3 gamma |
>> Learn More About Bispecific Antibody Targets and Services
1. Deng H, Niu Z, Zhang Z, et al. Back on the scene: Advances and challenges in CD3-related drugs in tumor therapy[J]. Drug Discovery Today, 2022, 27(8): 2199-2208. https://doi.org/10.1016/j.drudis.2022.04.019
2. Lee K J, Chow V, Weissman A, et al. Clinical use of blinatumomab for B-cell acute lymphoblastic leukemia in adults[J]. Therapeutics and clinical risk management, 2016: 1301-1310. https://doi.org/10.2147/TCRM.S84261
3. Kuhn C, Rezende R M, da Cunha A P, et al. Mucosal administration of CD3-specific monoclonal antibody inhibits diabetes in NOD mice and in a preclinical mouse model transgenic for the CD3 epsilon chain[J]. Journal of autoimmunity, 2017, 76: 115-122. https://doi.org/10.1016/j.jaut.2016.10.001
4. McAuley G E, Yiu G, Chang P C, et al. Human T cell generation is restored in CD3δ severe combined immunodeficiency through adenine base editing[J]. Cell, 2023, 186(7): 1398-1416. e23. https://doi.org/10.1016/j.cell.2023.02.027
This web search service is supported by Google Inc.
