 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> AGLink® Site-specific conjugation kit 
The conjugation method chosen for antibody-based conjugated drugs plays a pivotal role in determining the Antibody-Drug Ratio (DAR) and the uniformity of the resulting product, directly impacting its efficacy, tolerability, and stability.
Conjugation methods are commonly categorized as random or site-specific. Site-specific conjugation is increasingly preferred for its ability to yield conjugated drugs with high uniformity and enhanced safety profiles.
Almost all monoclonal antibodies are glycosylated at (or around) Asn-297 of the Fc domain. Glycan -mediated conjugation, a well-established site-specific method, utilizes the N297 glycosylation sites of the antibody for conjugation.
Being distant from the antigen-binding region, conjugation at this site preserves the antibody's binding function, minimizing the risk of compromising its antigen-binding ability post-conjugation. This method eliminates the need for antibody engineering modifications, enhancing convenience, versatility, and significantly expediting early-stage research efficiency.
Through collaboration with Glyco-Therapy Biotechnology Co., Ltd., we have developed the AGLink® DAR2&4 site-specific conjugation kit. Based on Glyco-therapy's YTConju™ conjugation platform, the kit facilitates efficient conjugation of various payloads, including toxin (MMAE), reactive functional groups (Tz/DBCO), and detection labels (Biotin). Designed to empower early-stage research and biological experiments in the field of conjugated drugs.
![]() One-pot, One step method
One-pot, One step method
![]() DAR 2 or DAR 4 kits available with one kit
DAR 2 or DAR 4 kits available with one kit
![]() Achieve Uniform DAR values overnight to within 24 hours
Achieve Uniform DAR values overnight to within 24 hours
![]() Click chemistry ready
Click chemistry ready
![]() Homogeneous and stable products after conjugation
Homogeneous and stable products after conjugation
![]() Different types of conjugation kit to use
Different types of conjugation kit to use
For DAR4 Conjugation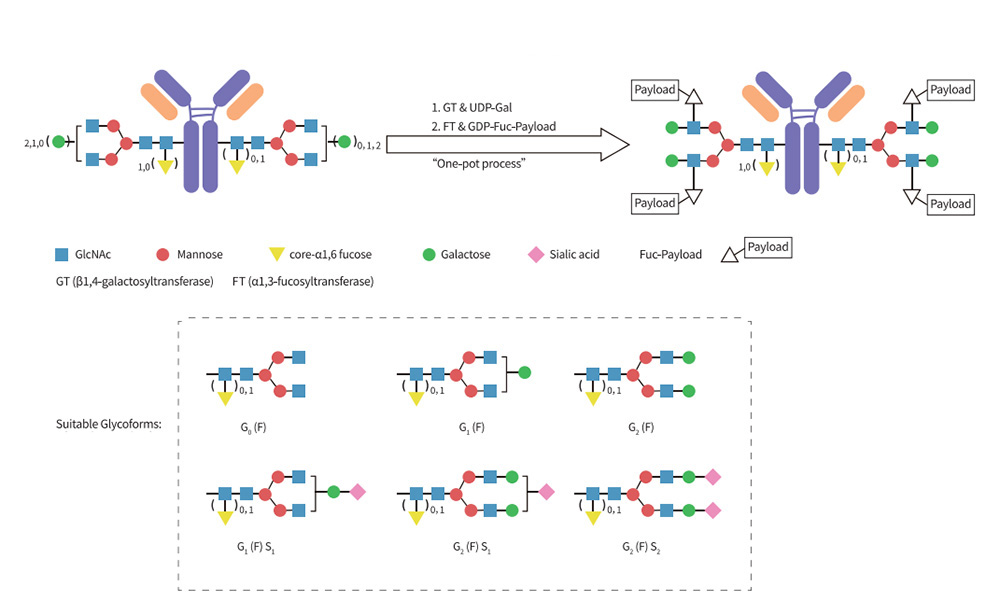
For DAR2 Conjugation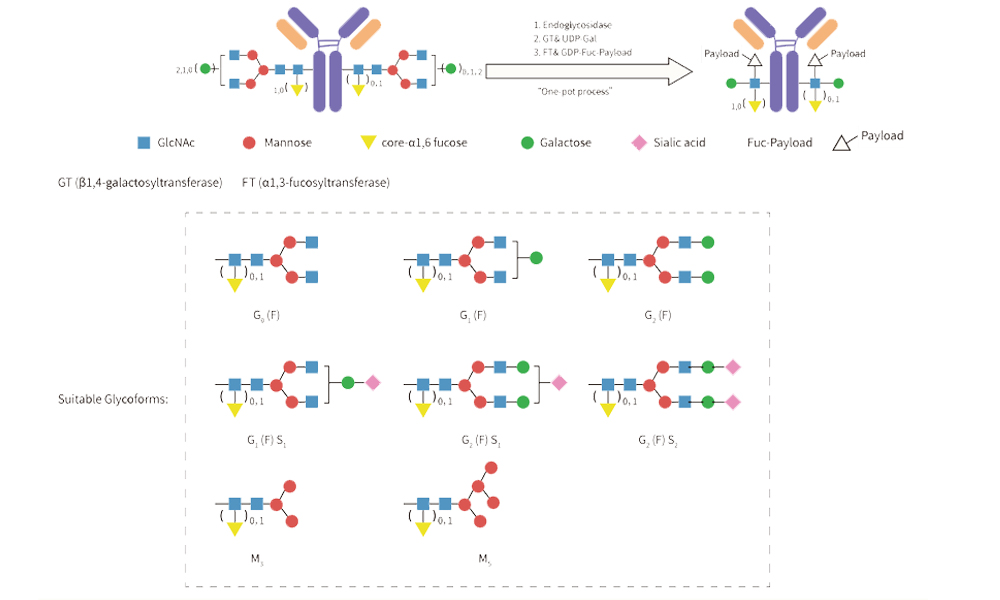
Toxin
Reactive Functional Group
Detection Label
Conjugation with MMAE for antibody screening, preparation, and performance studies in the ADC field.
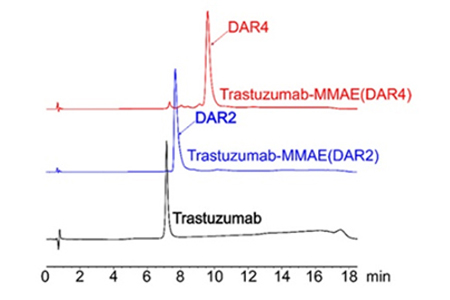
HIC-HPLC analysis of MMAE ADCs (DAR 4 & DAR 2)
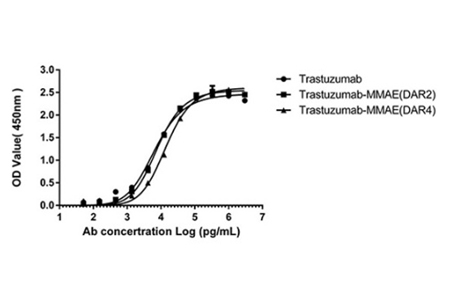
The analysis of the antigen-binding capacity of MMAE ADCs (DAR 4 & DAR 2), the results showing that binding to the HER2 antigen unaffected by the AGLink conjugation
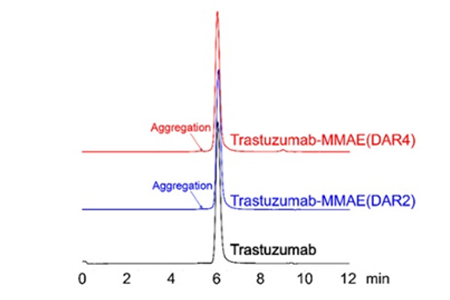
Less than 5% of antibody aggregation by SEC-HPLC
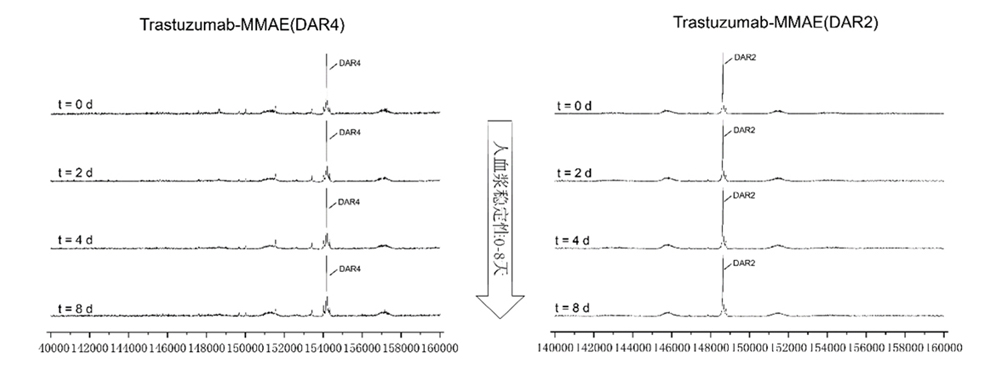
MMAE ADCs (DAR 4 & DAR 2) are stable in human plasma in vitro
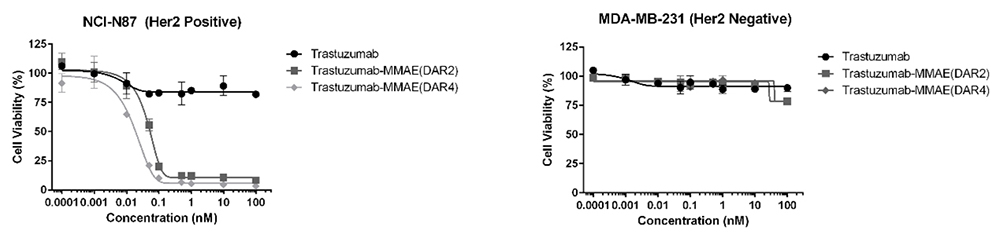
Validated in-vitro cell-killing activity of MMAE ADCs (DAR 4 & DAR 2)
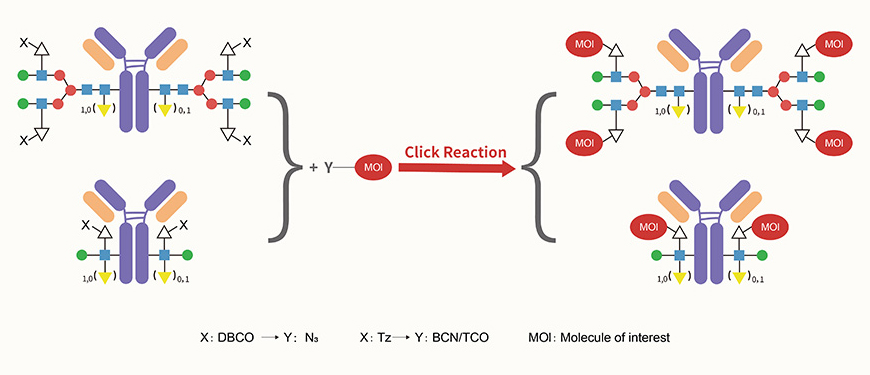
DBCO: DBCO reacts with N3-containing molecules in Click Chemistry, enhancing the hydrophilicity of the modified molecules.
Tetrazine: Tetrazine, reactive with BCN or TCO functional groups, demonstrates superior reactivity, making it better suited for conjugating complex molecules.
Utilizing click chemistry reactions enables the conjugation of a variety of moieties, including toxins, fluorescent dyes, oligonucleotides, nanobodies, and more.
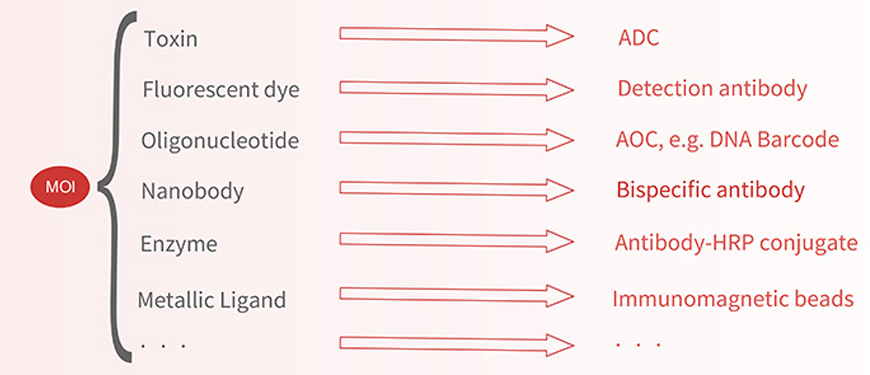
Screening and preparation of antibodies for detection or diagnosis
More homogeneous products and better batch-to-batch consistency compared to traditional chemical labeling
| Molecule | Cat. No. | Product Description | Preorder/Order |
|---|---|---|---|
| MMAE | ADC-P005 | AGLink® ADC Conjugation Kit (MMAE, DAR2&4, 1mg) | |
| Tetrazine | ADC-P006 | AGLink® ADC Conjugation Kit (Tetrazine, DAR2&4, 1mg) | |
| DBCO | ADC-P007 | AGLink® ADC Conjugation Kit (DBCO, DAR2&4, 1mg) | |
| Biotin | ADC-P008 | AGLink® ADC Conjugation Kit (Biotin, DAR2&4, 1mg) | |
| MMAE | ADC-P009 | AGLink® ADC Conjugation Kit (MMAE, DAR2&4, 5mg) | |
| Tetrazine | ADC-P010 | AGLink® ADC Conjugation Kit (Tetrazine, DAR2&4, 5mg) | |
| DBCO | ADC-P011 | AGLink® ADC Conjugation Kit (DBCO, DAR2&4, 5mg) | |
| Biotin | ADC-P012 | AGLink® ADC Conjugation Kit (Biotin, DAR2&4, 5mg) |
1. What is the minimum amount of antibody suitable for the AGLink® Conjugation Kit?
The minimum requirement is 200 μg, with an expected recovery rate exceeding 85%. To accommodate this quantity, scale down the reaction reagents proportionally according to the protocol provided.
2. Is it necessary to concentrate the antibody according to the protocol?
For optimal reaction efficiency, it is recommended to concentrate the antibody to the protocol’s suggested concentration. If concentration is not feasible, ensure that the antibody concentration remains above 1 mg/ml, and extend the reaction time to 48 hours to maintain conjugation efficiency.
3. Is this Conjugation Kit suitable for antibodies from all species?
This conjugation kit is highly effective for humanized antibodies conjugation. However, murine and rabbit antibodies, due to their complex structures and incomplete validation, may require an extended conjugation time of up to 48 hours to enhance conjugation efficiency.
4. Can the Linker in the AGLink® ADC Conjugation Kit be hydrolyzed, and if so, by what means?
The linker in the AGLink® ADC Conjugation Kit (MMAE, DAR 2 & 4, 1 mg & 5 mg) is vc-PAB, which can be cleaved by Cathepsin B. The cleavability of linkers in other kits depends on whether a cleavable linker is used during click chemistry conjugation.
5. How is the conjugation efficiency of the Conjugation Kit, and what methods are used to detect the Drug to Antibody Ratio (DAR) of conjugated product?
The conjugation efficiency of those kits can exceed 90%, and the DAR value can be analyzed using HIC-HPLC (MMAE) or LC-MS (MMAE/DBCO/Tetrazine/Biotin).
6. How to store the conjugated product and its stability under those conditions?
It is recommended to store the conjugated product in PBS buffer, where it can be preserved for over six months at -80°C. Avoid repeated freeze-thaw cycles.
7. What laboratory environment is required for this kit?
AGLink® conjugation kit can be used under normal laboratory conditions. Ensure you use personal protection equipment (lab coat, safety glasses, and chemical resistant nitrile gloves) while handling MMAE.
8. Are there any patent restrictions on this product, and will they impact on CMC development and commercial production?
The intellectual property of our AGLink® conjugation kit is covered in part or wholly by a patent owned or controlled by Glyco-therapy Biotechnology Co., Ltd. For more information about commercial rights, please contact inquiry@acrobiosystems.com for any comments or concerns.
9. If the Fc N297 site is mutated, can site-specific conjugation still be performed?
The current conjugation kit is designed for site-specific modification at glycosylated sites, if the N297 Fc glycosylation site is removed, this kit cannot be used. However, site-specific conjugation can be achieved using alternative methods.
This web search service is supported by Google Inc.
