 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> CMC Production Process of iPSC-based Cell Therapy 
Culture and Expansion of iPSCs
Genome Editing of iPSCs

Induced Differentiation of iPSCs
Culture and Expansion of iPSCs
 GMP Human Laminin 521 Protein GMP-Featured Product
GMP Human Laminin 521 Protein GMP-Featured Product
 Human Laminin 511 Protein, premium grade
Human Laminin 511 Protein, premium grade
 Human Vitronectin / VTN Protein
Human Vitronectin / VTN Protein

![]() Performance - facilitate rapid expansion of hPSC and support efficient differentiation into a diverse array of specialized cell types.
Performance - facilitate rapid expansion of hPSC and support efficient differentiation into a diverse array of specialized cell types.
![]() Better Adhesion - maintain good adhesion characteristics at a concentration as low as 2μg/ml.
Better Adhesion - maintain good adhesion characteristics at a concentration as low as 2μg/ml.
![]() Stemness Maintenance - no spontaneous differentiation is observed after several passages of hPSC culture.
Stemness Maintenance - no spontaneous differentiation is observed after several passages of hPSC culture.
![]() Lot-to-Lot Consistency - produced from a stable cell line, robust purification process, stringent QC.
Lot-to-Lot Consistency - produced from a stable cell line, robust purification process, stringent QC.
![]() Ready to Scale-up Supply - cGMP-compliant facility.
Ready to Scale-up Supply - cGMP-compliant facility.
![]() Supporting large-scale clinical supply - strictly adhere to the GMP management system, support multiple national regulations, and ensure stable supply for production.
Supporting large-scale clinical supply - strictly adhere to the GMP management system, support multiple national regulations, and ensure stable supply for production.
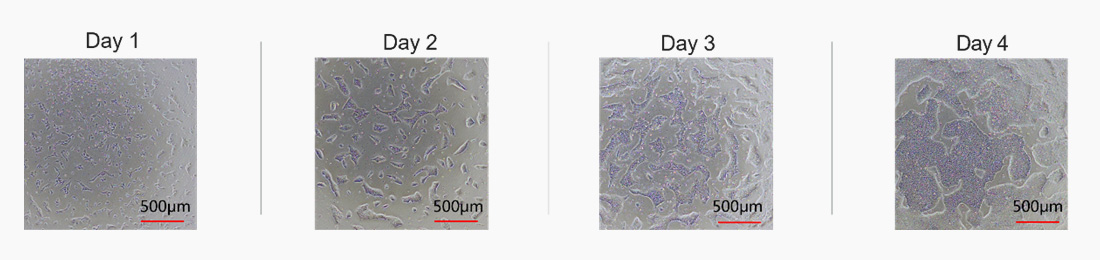
Laminin 521 (GMP-LA5H24) effectively maintains the expansion of human iPSCs.
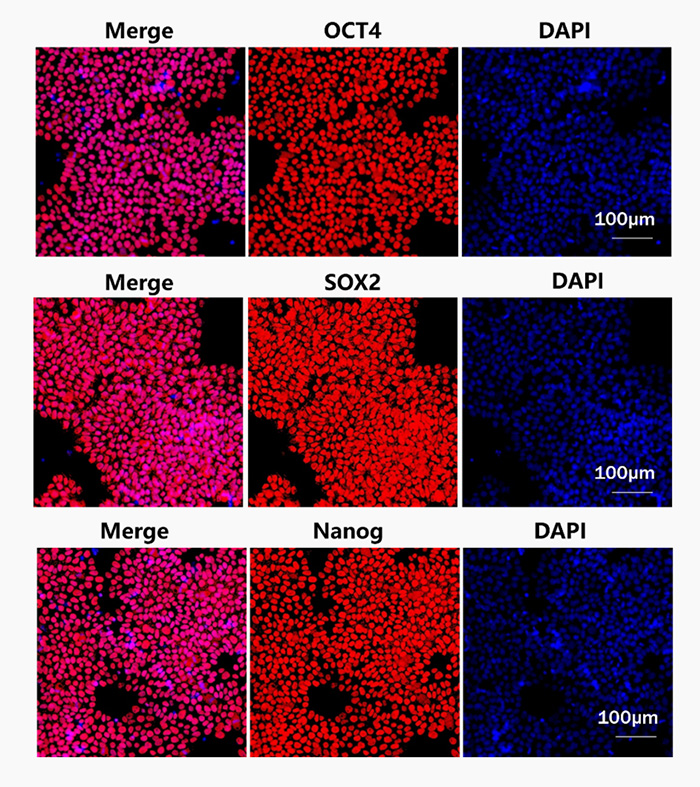
Laminin 521 (GMP-LA5H24) could maintain the stemness of iPSC after several passages
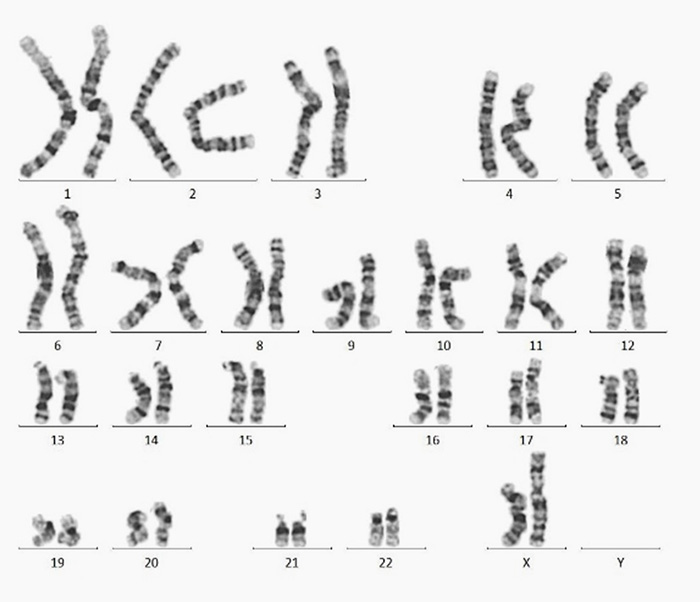
Normal karyotype (46, XX) was found in hiPSCs with Laminin 521(GMP-LA5H24) coating after 10 passages.
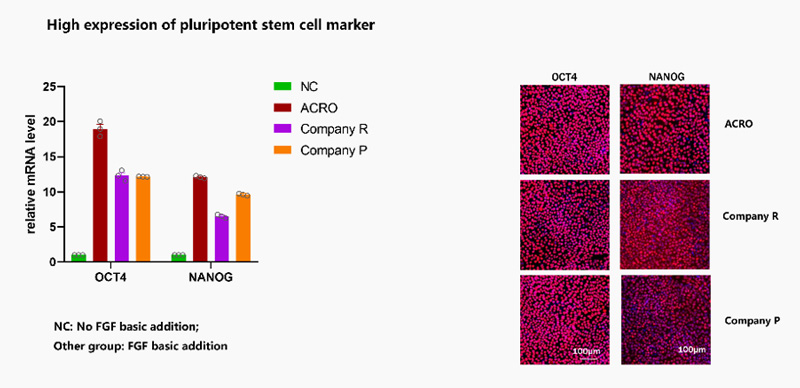
FGF basic (GMP-FGCH17) could highly support stemness maintenance in ESC/iPSC compared to other companies.
Genome Editing of iPSCs
![]() High purity, high enzyme activity, high cleavage efficiency
High purity, high enzyme activity, high cleavage efficiency
![]() Possesses nuclear localization signals to enhance editing efficiency
Possesses nuclear localization signals to enhance editing efficiency
![]() Aseptic, ultra-low endotoxin
Aseptic, ultra-low endotoxin
![]() Produced in GMP-compliant facilities and undergoes QC testing
Produced in GMP-compliant facilities and undergoes QC testing
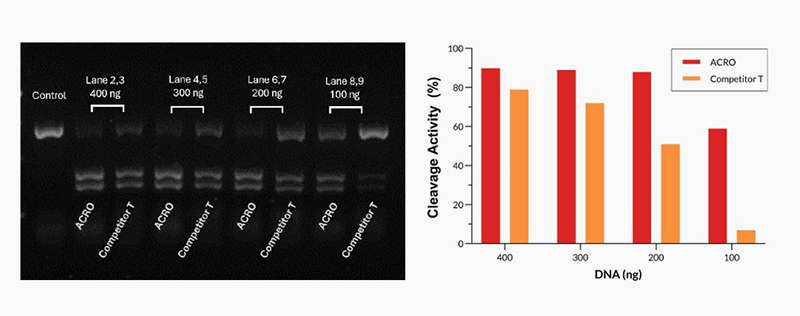
Different amounts of Cas9 were incubated with the same amount of excess gRNA and plasmid for 60 minutes at 37°C. When using 400-200 ng Acro Cas9, the cutting efficiency is greater than 90%. In comparison, when using a 200 ng Competitor T, the cutting efficiency is only about 50%.
Induced Differentiation of iPSCs
iPSC-T Cell
iPSC-NK Cell

iPSC-Neuron Cell
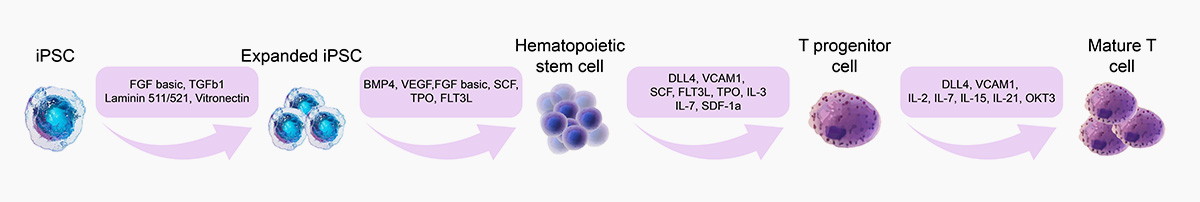
![]() GMP Grade DLL4, VCAM1:Eliminate the need of feeder cells in culture systems and support differentiation to T cells
GMP Grade DLL4, VCAM1:Eliminate the need of feeder cells in culture systems and support differentiation to T cells
![]() Validated activity by iPSC to T cell differentiation
Validated activity by iPSC to T cell differentiation
![]() Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
![]() Comprehensive quality release verification, with 16 quality control indicators
Comprehensive quality release verification, with 16 quality control indicators
![]() Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
![]() Pharmaceutical-grade production facility
Pharmaceutical-grade production facility
![]() Support for online and offline audits
Support for online and offline audits
![]() Completion of FDA Drug Master File (DMF) registration
Completion of FDA Drug Master File (DMF) registration
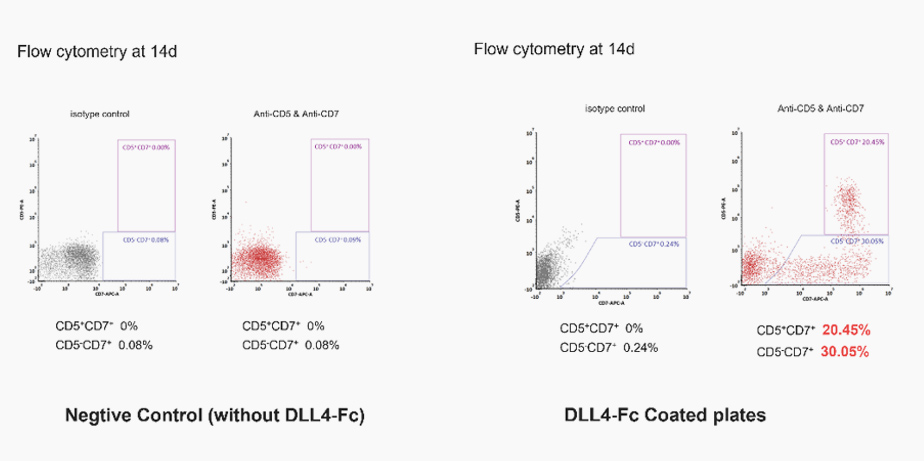
GMP Human DLL4 Protein, Fc Tag (GMP-DL4H28), together with SCF, TPO and other factors, could promote human CD34+CD45+ hematopoietic cells to differentiate into thymocyte (T) cell progenitors.
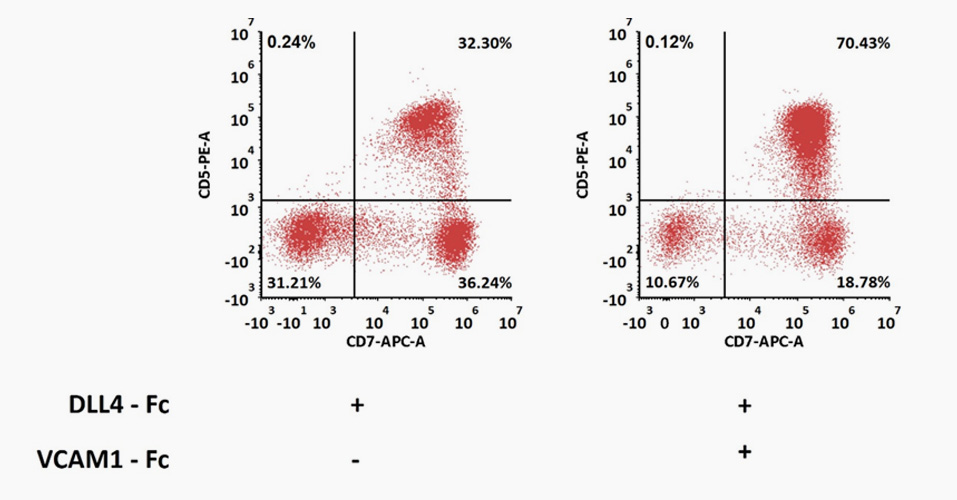
DLL4 (GMP-DL4H28) & VCAM1 (GMP-VC1H25) could highly support CD5+CD7+ T-cell progenitor differentiation from CD34+ HSPC.
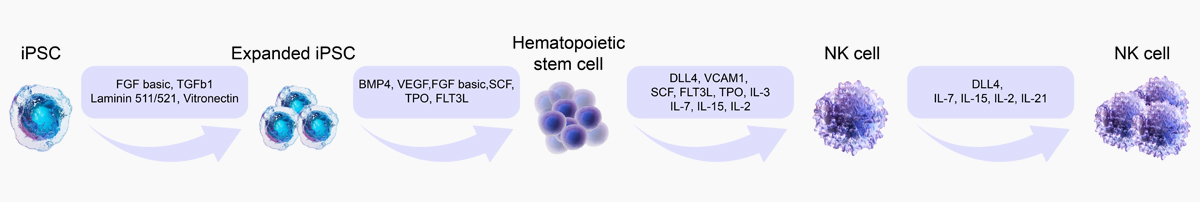
![]() GMP Grade DLL4, VCAM1:Eliminate the need of feeder cells in culture systems and support differentiation to NK cells
GMP Grade DLL4, VCAM1:Eliminate the need of feeder cells in culture systems and support differentiation to NK cells
![]() Validated activity by iPSC to NK cell differentiation
Validated activity by iPSC to NK cell differentiation
![]() Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
![]() Comprehensive quality release verification, with 16 quality control indicators
Comprehensive quality release verification, with 16 quality control indicators
![]() Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
![]() Pharmaceutical-grade production facility
Pharmaceutical-grade production facility
![]() Support for online and offline audits
Support for online and offline audits
![]() Completion of FDA Drug Master File (DMF) registration
Completion of FDA Drug Master File (DMF) registration
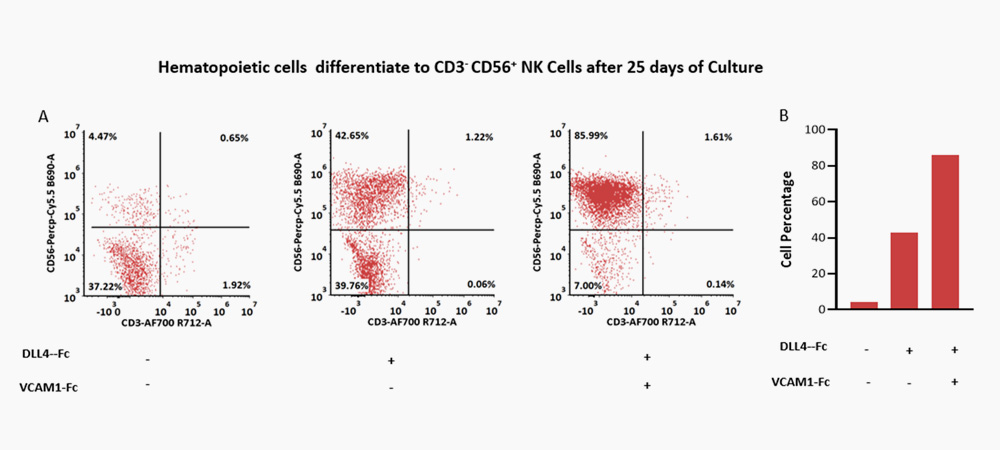
The combination of DLL4 (GMP-DL4H28) & VCAM1 (GMP-VC1H25) could significantly facilitate the differentiation efficiency of CD56+ CD3- NK cells.
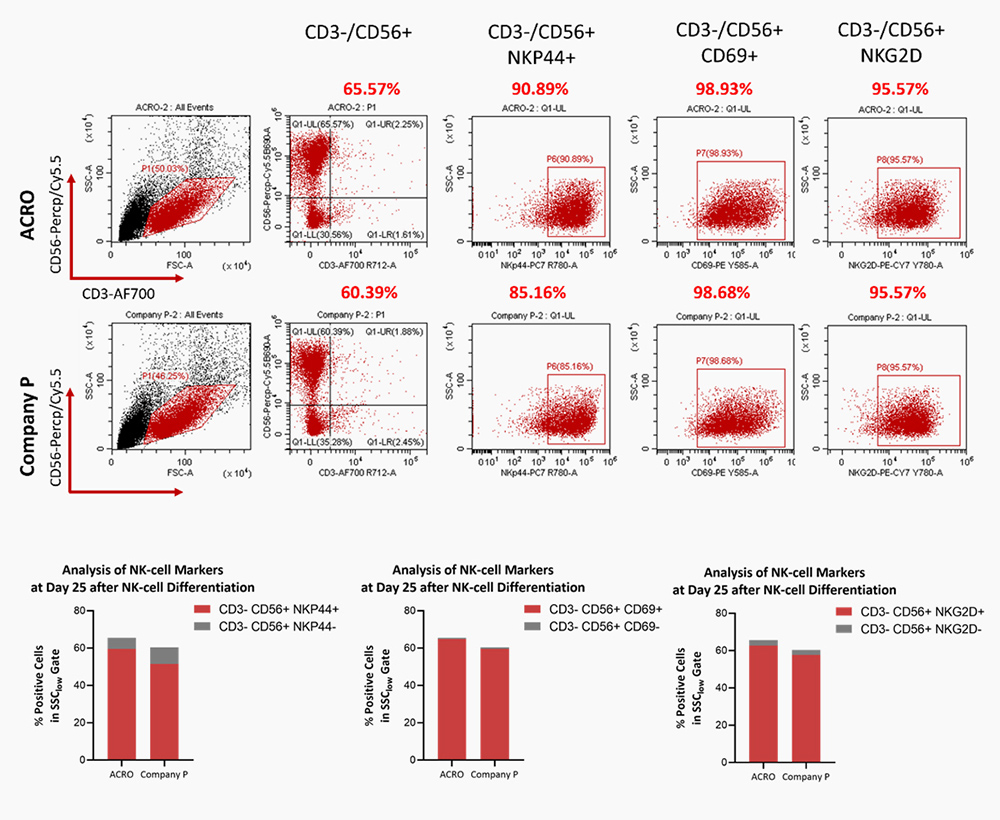
SCF(GMP-SCFH25), Flt3L(GMP-FLLH28), IL-7(GMP-L07H24) could significantly promote the HSPC differentiation to NK cells, comparable to Company P.
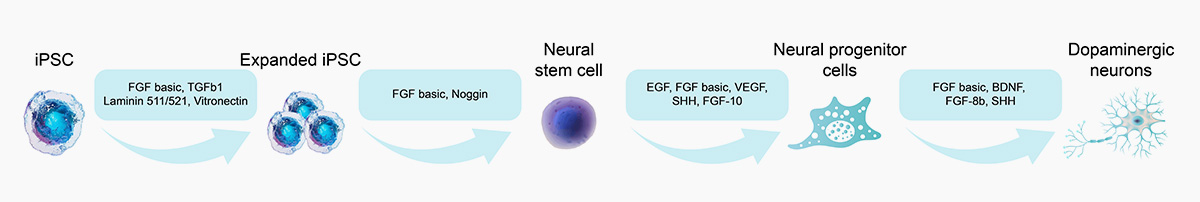
![]() GMP Grade FGF-8b: Efficiently induce the neuron progenitor cell into dopaminergic neurons differentiation
GMP Grade FGF-8b: Efficiently induce the neuron progenitor cell into dopaminergic neurons differentiation
![]() Validated activity by iPSC to neuron cell differentiation
Validated activity by iPSC to neuron cell differentiation
![]() Animal free production system and various impurities residual detection
Animal free production system and various impurities residual detection
![]() Sterile, no mycoplasma, no exogenous virus
Sterile, no mycoplasma, no exogenous virus
![]() High batch-to-batch consistency and stability
High batch-to-batch consistency and stability
![]() Support for online and offline audits
Support for online and offline audits
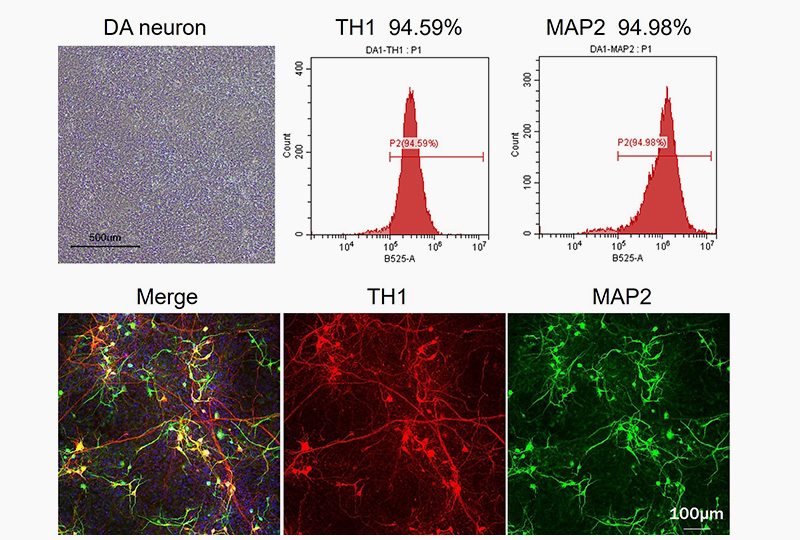
FGF8b (Cat. No. GMP-FGBH16) and Shh (Cat. No. SH7-H5229) could efficiently induce the neuron progenitor cell into dopaminergic neurons differentiation, highly expressed TH1 and MAP2 in immunofluorescence staining and FACS.
Resources
Cytokines residue ELISA kits
IL-2, IL-4, IL-6, IL-7, IL-10, IL-15, IL-21, IL-1B, TNF-alpha, GM-CSF residue kits.
HCD detection
Enzyme residue kit
DNase Activity Assay Kit (Fluorescence)
Mono-growth factor detection
Multiplex detection
This web search service is supported by Google Inc.
