 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > Human FcRn Binding Kit (TR-FRET) - Your Powerful Ally for High-Throughput Antibody Screening 
Since the successful development of humanized antibodies in the 1980s, antibody-based drugs have played an increasingly important role in clinical treatment, leveraging their advantages of low immunogenicity and sustainable dosing.
To date, more than 100 monoclonal antibodies (mAbs) and derived therapeutics have been approved for disease treatment. Among these antibody-derived products, key formats include Fc fusion proteins and antibody-drug conjugates (ADCs).
These novel antibody-based therapeutics not only retain the targeting specificity inherent to antibodies, but also incorporate other biologically active components, further enhancing their therapeutic potential. In particular, Fc fusion proteins, with their unique structural and functional properties, have demonstrated vast application potential in the treatment of autoimmune disorders. Representative products such as etanercept, alefacept, and abatacept have provided patients with additional effective treatment options. The remarkable advancements in antibody technology have revolutionized the biopharmaceutical landscape, offering new hope for patients afflicted by a wide range of diseases.
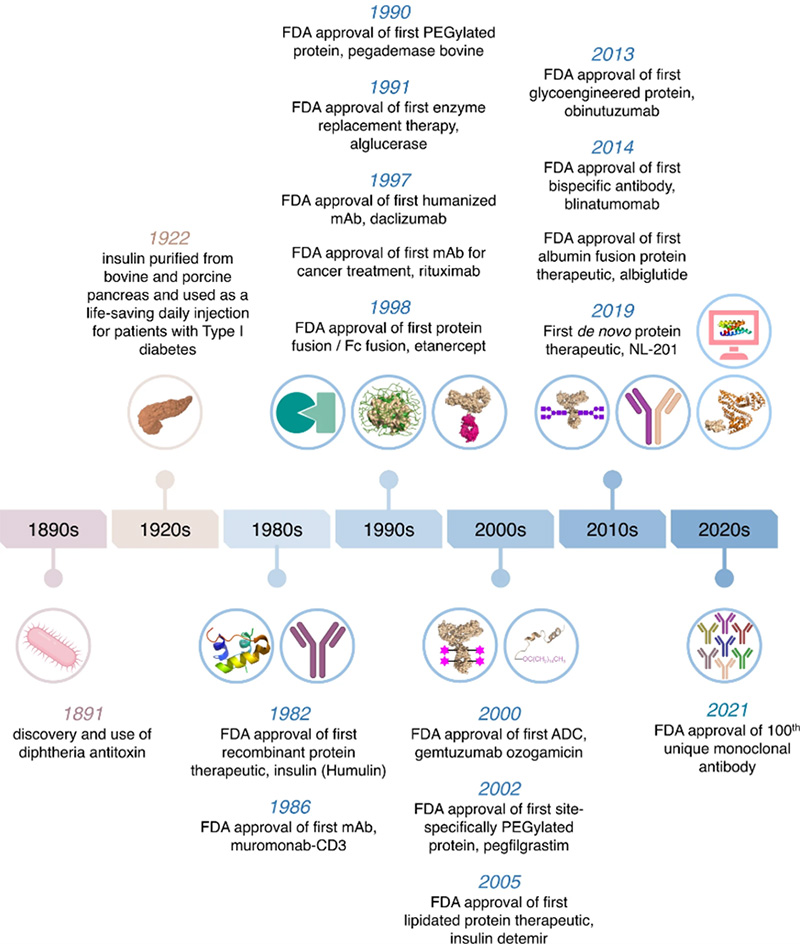
Milestones in protein and antibody-based drug development since 1891. As early as 1998, the biopharmaceutical landscape witnessed a groundbreaking milestone with the approval of the first Fc fusion protein drug by the US FDA – Etanercept. Etanercept, a fusion of the extracellular domain of human TNF receptor 2 (CD120) and the Fc region of human IgG1, revolutionized the treatment of autoimmune disorders such as rheumatoid arthritis.
Data shows that the global Fc fusion protein drug market reached approximately $30.67 billion in 2023. It is projected to soar to $ 62.1 billion by 2030, with a compound annual growth rate (CAGR) of 10.7% from 2024 to 2030. This significant growth highlights the vast potential and expanding role of Fc fusion proteins in the treatment of autoimmune diseases.
The half-life of Fc fusion proteins and antibody drugs is a crucial consideration in their development process. In healthy individuals, the serum half-life of IgG1 is approximately 21 days, a characteristic closely associated with the interaction between the neonatal Fc receptor (FcRn) and IgG. This interaction is pivotal for extending the circulating time of these therapeutic proteins, thereby enhancing their therapeutic efficacy and reducing the frequency of administration.
In the pursuit of developing highly effective and reliable antibody-based therapeutics, researchers have established a diverse array of in vitro methods to detect and characterize the FcRn - IgG interactions. These screening techniques can be broadly categorized into two main classes: cell-based and non-cell-based approaches:
1. Cell-based detection methods: Flow cytometry - directly interrogates the binding activity between cell surface-expressed FcRn and labeled IgG, providing a physiologically relevant assessment of the receptor-ligand interactions.
2. Non-cell-based detection methods: Including Surface Plasmon Resonance (SPR), Biolayer Interferometry (BLI), Homogeneous Time-resolved Fluorescence (HTRF) and Amplified Luminescent Proximity Homogeneous Assay (AlphaLISA). These methods evaluate the binding characteristics of FcRn proteins to IgG under different pH conditions, such as in solution or on artificial solid surfaces.
Different detection methods each have their own strengths and weaknesses in terms of sensitivity, precision, reproducibility, time, and cost. Fluorescence Resonance Energy Transfer (FRET) theory was first proposed in 1948 and experimentally validated in 1967. This technique can measure molecule interactions within a distance of 1.0-6.0 nm. Through continuous exploration by scientists, FRET technology has become a crucial molecular biology detection method due to its high sensitivity, simplicity, and broad application range. It has been successfully employed in the screening of antibody drug candidates.
Based on Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) technology and the competitive binding mechanism of human IgG antibodies to FcRn, ACROBiosystems proudly presents the new product: Human FcRn Binding Kit (TR-FRET) (Cat. No. FRT-01).
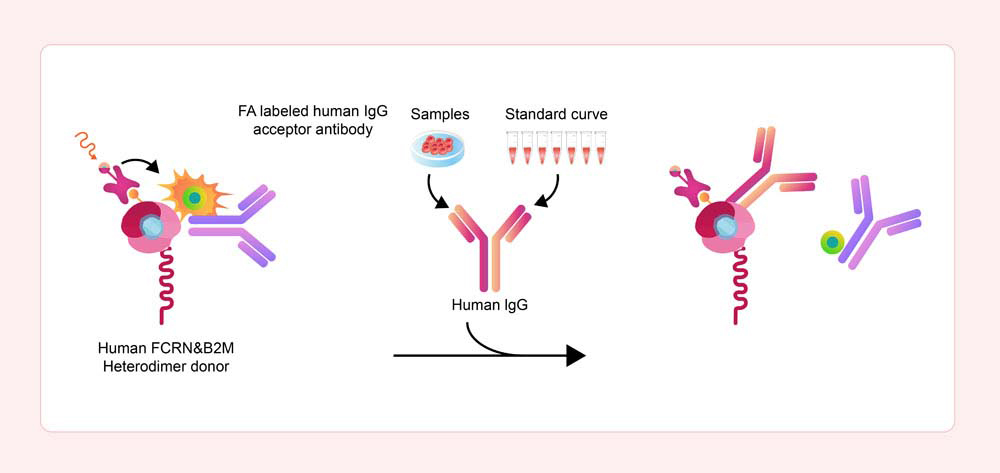
Principle of the Human FcRn Binding Kit (TR-FRET)
In the Human FcRn Binding Kit (TR-FRET), the donor is a mixture of biotinylated recombinant human FcRn and europium-chelate labeled streptavidin (SA), while the acceptor is a specific FA-labeled human IgG1 antibody.
When the sample does not contain any other components that can bind to FcRn, the donor and acceptor molecules will bind closely together. The donor's emission spectrum partially overlaps with the acceptor's excitation spectrum, allowing for efficient FRET to occur.
Upon excitation at a specific wavelength, the donor will emit a 620 nm signal, which is then captured by the acceptor, causing it to emit a 665 nm signal. This effective FRET signal is directly proportional to the extent of FcRn-IgG complex formation.
However, if the sample contains the components that can bind to the donor, they will block the binding between the donor and acceptor, preventing the FRET from occurring. This results in a decrease in the detected signal.
"Precision and Reliability" - High detection sensitivity, accurate results, and minimal matrix effects, ensuring reliable and trustworthy data.
"Slick and Streamlined" - Eliminating washing steps, simple and expedited workflow, and significantly reducing the time required to obtain results.
"Unwavering Consistency" - Stringent quality control of raw materials, high batch-to-batch consistency, and stable supply.
• Typical IgG Antibody Binding Data
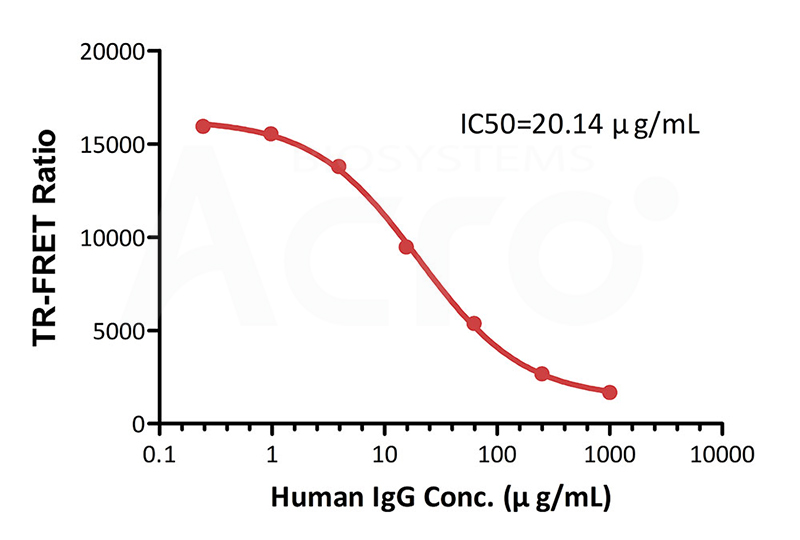
Inhibition of Europium-chelate labeled FcRn:
FA labeled human IgG antibody binding by Human IgG
Premix serial dilutions of Human IgG standard (Catalog # FRT01-C03) (1:4 serial dilution, from 1000 μg/mL to 0.24 μg/mL (6666.67-1.63 nM)) and Human FCRN&B2M Heterodimer Protein Europium-chelate (Catalog # FRT01-C01) and incubate at room temperature (20℃-25℃) for 0.5 hour. Then add FA labeled human IgG antibody (Catalog # FRT01-C02) and incubate at room temperature (20℃-25℃) for 0.5 hour. Detection was performed with IC50 of 20.14 μg/mL. The assay was performed according to the above-described Datasheet (QC tested).
• IgG isoforms Binding to FcRn
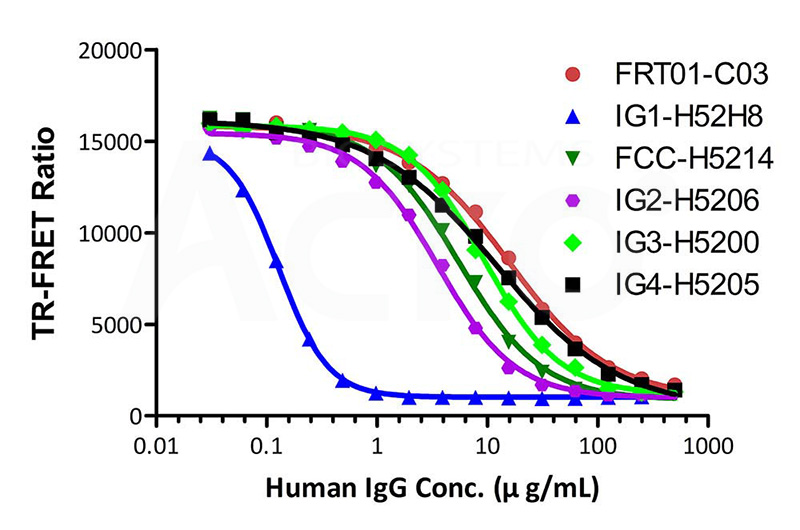
The kit (Cat. No. FRT-01) has been used to detect different human IgG isoforms of Human IgG1, Human IgG2, Human IgG3, Human IgG4, and modified Human IgG1 Fc (C103S, M135Y, S137T, T139E, H316K, N317F) Protein, His Tag (Cat. No. IG1-H52H8) with different affinities binding to FcRn.
Sparking a Powerful Synergy: When the high-sensitivity TR-FRET technology collides with ACRO's high quality FcRn proteins, the result is the reliable Human FcRn Binding Kit (TR-FRET) (Cat. No. FRT-01), the perfect choice for high-throughput screening of antibody drug.
1. Liu L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell. 2018 Jan;9(1):15-32. doi: 10.1007/s13238-017-0408-4.
2. Ebrahimi SB, Samanta D. Engineering protein-based therapeutics through structural and chemical design. Nat Commun. 2023 Apr 27;14(1):2411. doi: 10.1038/s41467-023-38039-x.
3. Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, Yamaguchi T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol. 2010 Feb 15;184(4):1968-76. doi: 10.4049/jimmunol.0903296.
4. Song Y, Deng X, Shi W, Tang F, Huang W, Gong L, Qin Q. A homogeneous time-resolved fluorometric energy transfer assay for the binding assessment of FcRn with IgG antibodies. J Immunol Methods. 2021 Dec;499:113180. doi: 10.1016/j.jim.2021.113180.
This web search service is supported by Google Inc.
