 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!

1e6 of the anti-SLAMF7 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human SLAMF7, His Tag (Cat. No. SL7-HP2H3) and negative control protein respectively, PE signals was used to evaluate the binding activity (QC tested).
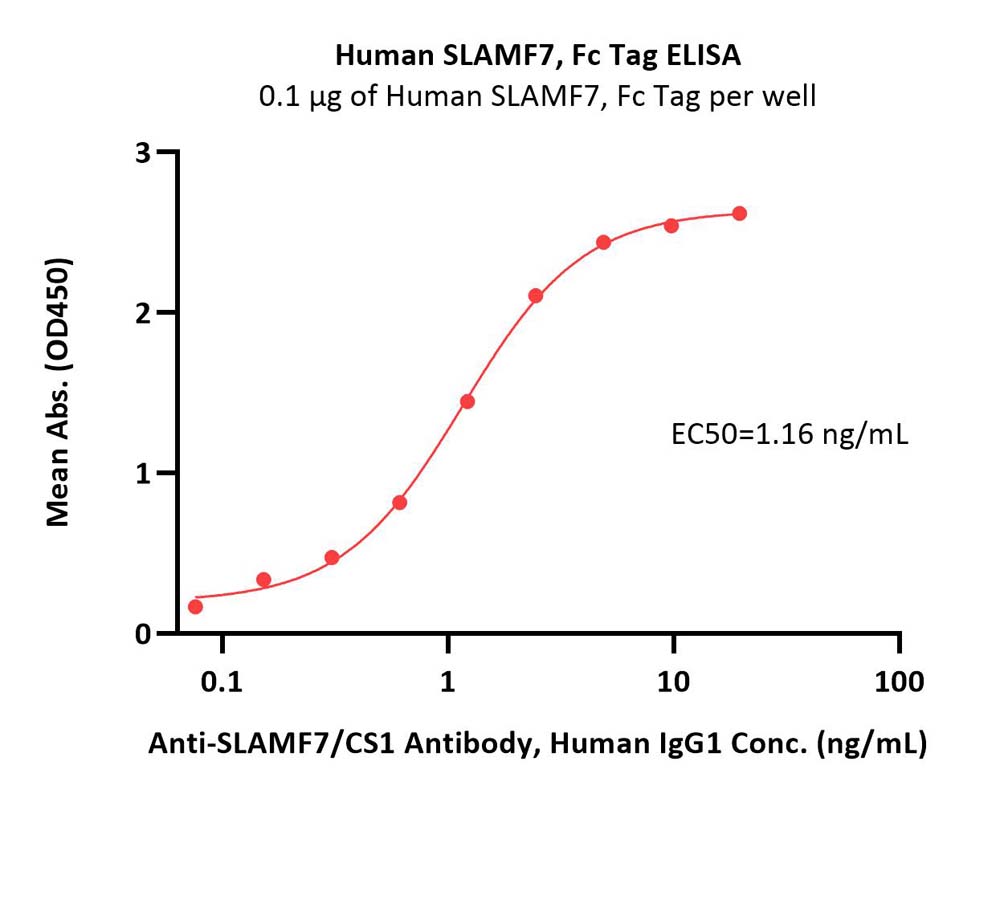
Immobilized Human SLAMF7, Fc Tag (Cat. No. SL7-H5256) at 1 μg/mL (100 μL/well) can bind Anti-SLAMF7/CS1 Antibody, Human IgG1 with a linear range of 0.2-2 ng/mL (QC tested).
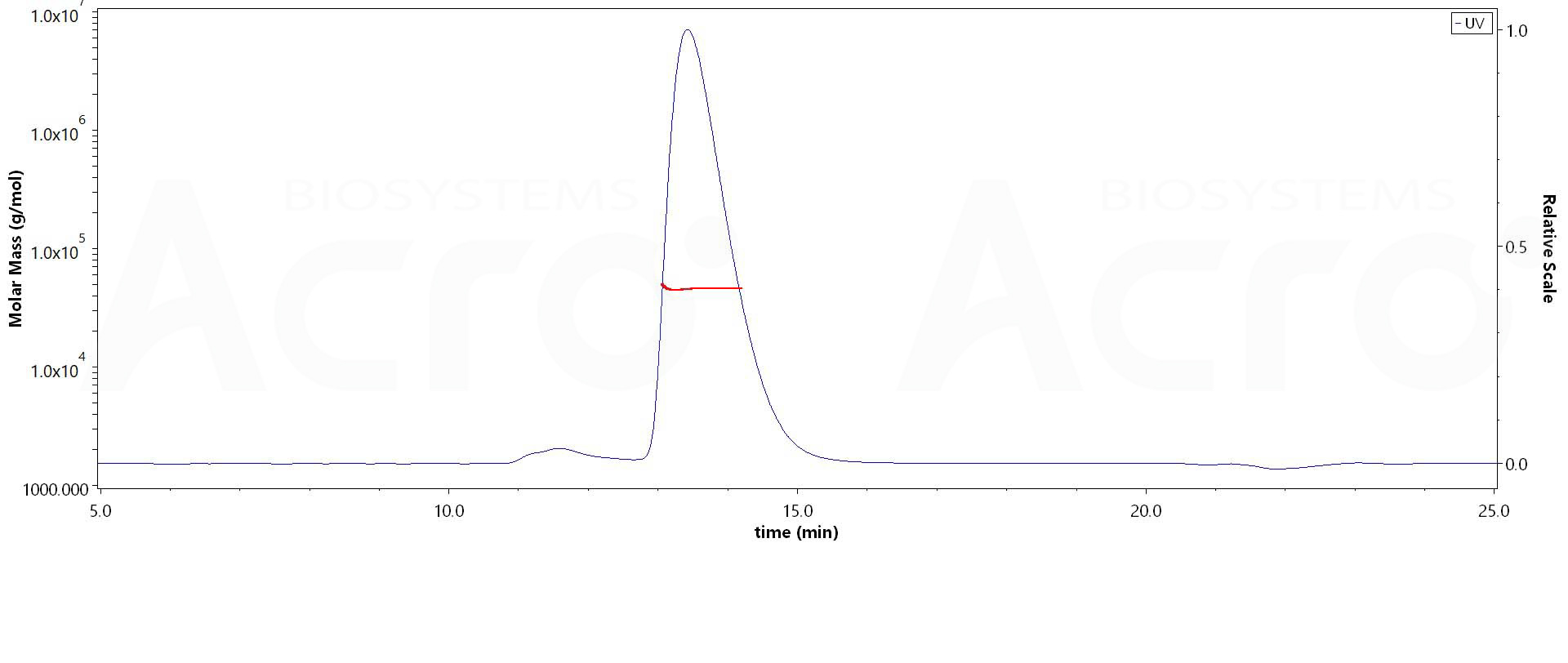
The purity of Rhesus macaque SLAMF7, His Tag (Cat. No. SL7-R52H7) is more than 90% and the molecular weight of this protein is around 40-50 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Elotuzumab | PDL-063; HuLuc-63; BMS-901608 | Approved | Abbvie Inc, Bristol-Myers Squibb Company | Empliciti | United States | Multiple Myeloma | Bristol-Myers Squibb Company | 2015-11-30 | Hematologic Neoplasms; Hematopoietic stem cell transplantation (HSCT); Polycythemia Vera; Smoldering Multiple Myeloma; Leukemia, Plasma Cell; Multiple Myeloma; Primary Myelofibrosis; Lymphoma; Thrombocythemia, Essential | Details |
| Elotuzumab | PDL-063; HuLuc-63; BMS-901608 | Approved | Abbvie Inc, Bristol-Myers Squibb Company | Empliciti | United States | Multiple Myeloma | Bristol-Myers Squibb Company | 2015-11-30 | Hematologic Neoplasms; Hematopoietic stem cell transplantation (HSCT); Polycythemia Vera; Smoldering Multiple Myeloma; Leukemia, Plasma Cell; Multiple Myeloma; Primary Myelofibrosis; Lymphoma; Thrombocythemia, Essential | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| SLAMF7 CAR-T cell therapy (Wuerzburg University Hospital) | Phase 2 Clinical | University Hospital Würzburg | Multiple Myeloma | Details | |
| WS-CART-CS1 | WS-CART-CS1 | Phase 1 Clinical | Washington University School Of Medicine, Paula C. & Rodger O. Riney Blood Cancer Research | Multiple Myeloma | Details |
| MB-104 | MB-104; MB-105; MB-104 CS1 CAR; MB-105 PSCA CAR | Phase 1 Clinical | City Of Hope National Medical Center | Stomach Neoplasms; Glioblastoma; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Prostatic Neoplasms | Details |
| CS1 Targeted CAR T-cells (Yake Biotechnology) | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd | Multiple Myeloma | Details | |
| CD319 chimeric antigen T cell therapy (Wuhan Bio Raid Biotechnology) | Clinical | Wuhan BioRaid Biotechnology Co Ltd, Tongji Hospital | Hematologic Neoplasms | Details | |
| SLAMF7 CAR-T cell therapy (Wuerzburg University Hospital) | Phase 2 Clinical | University Hospital Würzburg | Multiple Myeloma | Details | |
| WS-CART-CS1 | WS-CART-CS1 | Phase 1 Clinical | Washington University School Of Medicine, Paula C. & Rodger O. Riney Blood Cancer Research | Multiple Myeloma | Details |
| MB-104 | MB-104; MB-105; MB-104 CS1 CAR; MB-105 PSCA CAR | Phase 1 Clinical | City Of Hope National Medical Center | Stomach Neoplasms; Glioblastoma; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Prostatic Neoplasms | Details |
| CS1 Targeted CAR T-cells (Yake Biotechnology) | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd | Multiple Myeloma | Details | |
| CD319 chimeric antigen T cell therapy (Wuhan Bio Raid Biotechnology) | Clinical | Wuhan BioRaid Biotechnology Co Ltd, Tongji Hospital | Hematologic Neoplasms | Details |
This web search service is supported by Google Inc.
