 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| A2aR/A2bR Antagonist | Small molecule | Oncology/Cancer | Advanced solid tumor,Advanced hematological tumor | IND | Global (except China) |
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| ADA-H52D3 | Human | Human ADORA2A Protein, Flag,His Tag (Detergent) |
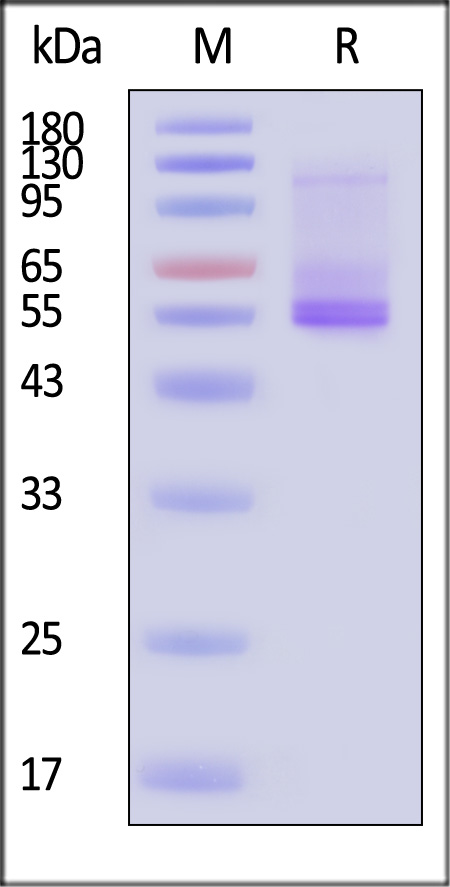
|
|
|
| ADA-H52P4 | Human | Human ADORA2A Full Length Protein (VLP) |

|

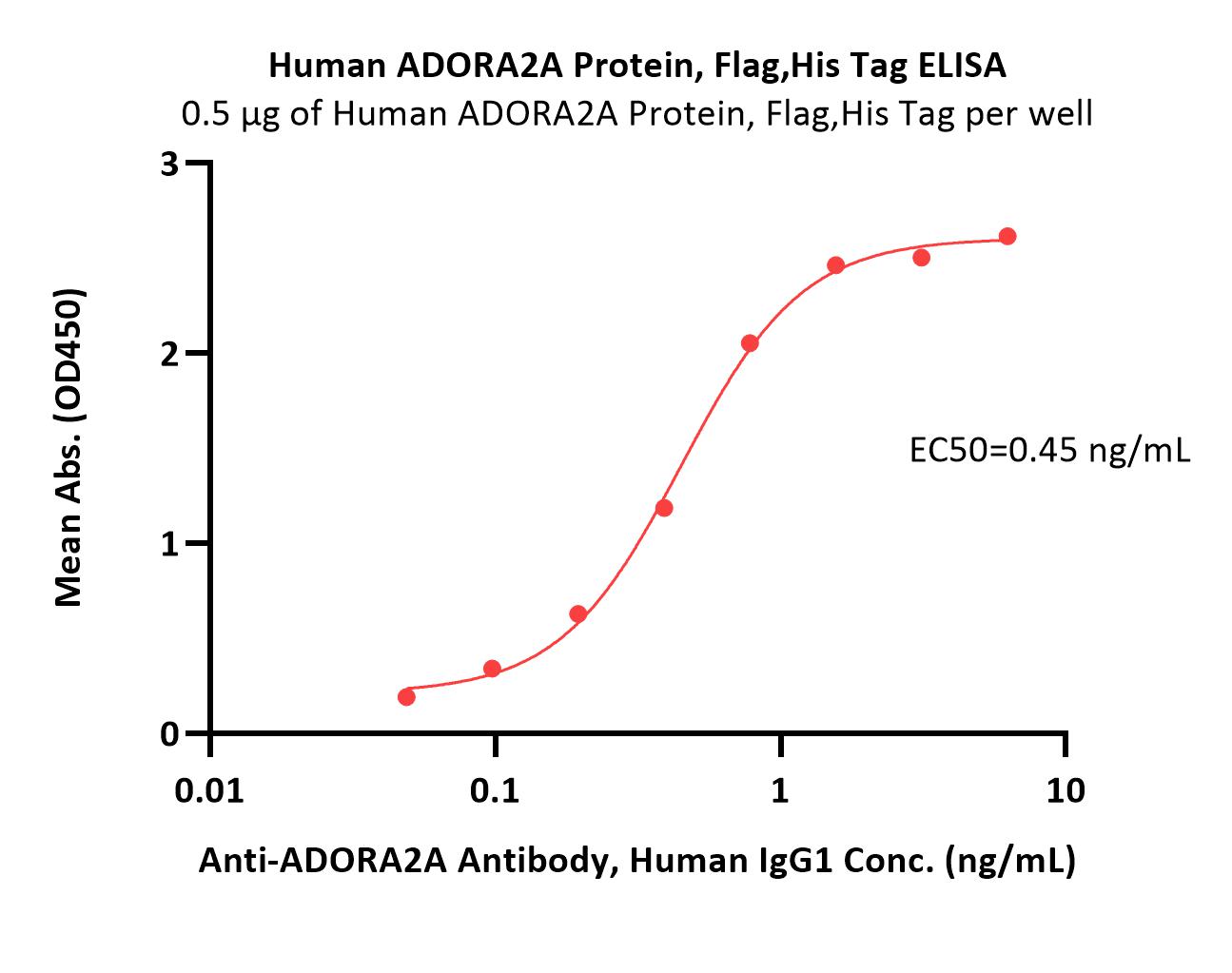
Immobilized Human ADORA2A Full Length Protein-VLP (Cat. No. ADA-H52P4) at 5 μg/mL (100 μL/well) can bind Monoclonal Anti-Human ADORA2A antibody, Human IgG1 with a linear range of 0.3-5 ng/mL (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Regadenoson Hydrate | CVT-3146 | Approved | Astellas Pharma Inc | Lexiscan, Rapiscan | United States | Contrast agents | Astellas Pharma Inc | 2008-04-10 | Myocardial Ischemia; Rejection of lung transplantation; Cardiovascular contrast agents; Pneumonia; Coronary Artery Disease; Heart Diseases; Glioblastoma; Coronavirus Disease 2019 (COVID-19); Retinal Artery Occlusion; Oligodendroglioma; Contrast agents; Cardiovascular Diseases; Astrocytoma; Diagnostic agents; Glioma; Anemia, Sickle Cell | Details |
| Caffeine/Ergotamine Tartrate | Approved | Novartis Pharma Ag | Cafergot | Mainland China | Migraine Disorders | Tianjin Medical University Pharmaceutical Factory | 1948-11-26 | Migraine Disorders | Details | |
| Oxtriphylline | Approved | Theocolin | Canada | null | Asthma | Details | ||||
| Caffeine/Metamizole Sodium/Dihydroergotamine Mesylate | Approved | Cefaliv | Migraine Disorders | Details | ||||||
| Adenine | Approved | Leucon | Japan | Leukopenia | Ohara Pharmaceutical Co Ltd | Leukemia; Leukopenia | Details | |||
| Istradefylline | KW-6002 | Approved | Kyowa Hakko Kirin Co Ltd | Nourianz, Nouriast | Japan | Parkinson Disease | Kyowa Hakko Kirin Co Ltd | 2013-03-25 | Substance-Related Disorders; Restless Legs Syndrome; Sleep Wake Disorders; Parkinson Disease; Cognitive Dysfunction; Hepatic Insufficiency; Movement Disorders; Amyotrophic Lateral Sclerosis | Details |
| Acetaminophen/Methylephedrine Hydrochloride/Dextromethorphan Hydrobromide/Chlorphenamine Maleate/Caffeine/Sulfogaiacol | Approved | Mainland China | Common Cold | Zhejiang Apeloa Kangyu Pharmaceutical Co Ltd | Common Cold | Details | ||||
| Dextromethorphan Hydrobromide/Chlorphenamine Maleate/Sodium Citrate/Ammonium Chloride/Caffeine | Approved | 尔可芬 | Cough | Details | ||||||
| Acetaminophen/Caffeine/Codeine Phosphate/Diphenhydramine Hydrochloride | Approved | 斯力帮 | Headache | Details | ||||||
| Ferric Ammonium Citrate/Vitamin B1/Sodium glycerophosphate/Caffeine | Approved | Ischemia | Details | |||||||
| Norethidranum Dipropionas/Caffeine/Vitamin B6 | Approved | Mainland China | Contraception | Huazhong Pharmaceutical Co Ltd | 1981-01-01 | Contraception | Details | |||
| Acetaminophen/Propyphenazone/Caffeine | Approved | 散利痛, 散列通 | Common Cold | Details | ||||||
| Quinine Hydrochloride Hydrate/Caffeine | Approved | Malaria | Details | |||||||
| Dihydroergocryptine/Caffeine | Approved | 洛斯宝, VASOBRAL | Diabetic Retinopathy; Diabetes Mellitus | Details | ||||||
| Aminophenazone/Phenacetin/Caffeine | Approved | Pain; Fever | Details | |||||||
| Aminophenazone/Caffeine/Calcium Glycerophosphate | Approved | Headache; Dysmenorrhea; Neuralgia | Details | |||||||
| Acetaminophen/Caffeine/Chlorphenamine Maleate | Approved | Common Cold | Details | |||||||
| Aminophenazone/Caffeine/Chlorphenamine Maleate | Approved | Headache; Common Cold; Neuralgia | Details | |||||||
| Aspirin/Caffeine/Codeine Phosphate | Approved | 柯克 | Pain | Details | ||||||
| Acetaminophen/Pseudoephedrine Hydrochloride/Caffeine | Approved | Common Cold | Details | |||||||
| Aspirin/Acetaminophen/Caffeine/Chlorphenamine Maleate | Approved | Common Cold | Details | |||||||
| Aspirin/Caffeine/Chlorphenamine Maleate/Tartaric Acid | Approved | Common Cold | Details | |||||||
| Acetaminophen/Caffeine/Pseudoephedrine Hydrochloride/Chlorphenamine Maleate | Approved | Common Cold | Details | |||||||
| Aminophenazone/Phenacetin/Caffeine/Phenobarbital | Approved | Migraine Disorders | Details | |||||||
| Acetaminophen/Chlorphenamine Maleate/Caffeine/Guaifenesin | Approved | Common Cold | Details | |||||||
| Acetaminophen/Amantadine Hydrochloride/Caffeine/Artificial Bezoar | Approved | Common Cold | Details | |||||||
| Acetaminophen/Salicylamide/Pseudoephedrine Hydrochloride/Caffeine/Triprolidine Hydrochloride Hydrate | Approved | 联邦菲迪乐 | Common Cold | Details | ||||||
| Sodium Benzoate/Caffeine | Approved | Central Nervous System Diseases | Details | |||||||
| Aminophenazone/Caffeine | Approved | Fever | Details | |||||||
| Antipyrine/Caffeine | Approved | Common Cold | Details | |||||||
| Aspirin/Caffeine | Approved | Common Cold | Details | |||||||
| Antipyrine/Caffeine/Citric Acid | Approved | 米格来宁 | Mainland China | Sedation | Xi'An Disai Biological Pharmaceutical Co Ltd, Heilongjiang Hasing Pharmaceutical Group Co Ltd, Huadong Medicine (Xi'An) Bodyguard Pharmaceutical Co Ltd, Mudanjiang Lingtai Medicdment Co Ltd | 1982-01-01 | Sedation | Details | ||
| Aminophenazone/Acetaminophen/Caffeine/Chlorphenamine Maleate | Approved | 克感敏, 扑感敏, 朴感敏 | Common Cold | Details | ||||||
| Acetaminophen/Caffeine/Ephedrine Hydrochloride/Diphenhydramine Hydrochloride | Approved | Influenza, Human | Details | |||||||
| Acetaminophen/Caffeine/Chlorphenamine Maleate/Artificial Bezoar | Approved | Common Cold | Details | |||||||
| Aspirin/Phenacetin/Caffeine | Approved | Headache; Dysmenorrhea; Toothache; Neuralgia; Fever; Arthralgia | Details | |||||||
| Aspirin/Caffeine/Acetaminophen | Approved | United States | Migraine Disorders | Haleon PLC | Migraine Disorders; Anesthesia | Details | ||||
| Acetaminophen/Butalbital/Caffeine | Approved | Headache | Details | |||||||
| Acetaminophen/Caffeine | Approved | Zentiva As | Mainland China | Pain | Sino--American Shanghai Squibb Pharmaceutical Ltd | Pain | Details | |||
| Sodium glycerophosphate/Caffeine/Vitamin B/Phosphoric Acid/Niacin | Approved | Details | ||||||||
| Acetaminophen/Caffeine/Phenylephrine Hydrochloride/Chlorphenamine Maleate/Vitamin B | Approved | Details | ||||||||
| Acetaminophen/Chlorphenamine Maleate/Methylephedrine Hydrochloride/Sulfogaiacol/Caffeine | Approved | Mainland China | Common Cold | Hainan Sanfengyou Pharmaceutical Co Ltd | 2006-05-16 | Common Cold | Details | |||
| Brompheniramine Maleate/Bromhexine Hydrochloride/Caffeine/Acetaminophen/Phenylephrine Hydrochloride | Approved | Common Cold | Details | |||||||
| Acetaminophen/Amantadine Hydrochloride/Caffeine/Chlorphenamine Maleate/Artificial Bezoar | Approved | 复方氨酚烷胺 | Mainland China | Common Cold | null | 1984-01-01 | Common Cold | Details | ||
| Caffeine | Approved | Fatigue; Mental Fatigue; Memory Disorders; Cocaine-Related Disorders; Sexual Dysfunction, Physiological; Parkinson Disease; Cognitive Dysfunction; Diabetes Complications; Diabetic Nephropathies; Postoperative Cognitive Complications; Diabetes Mellitus, Type 1; Delirium; Apnea; Disorders of Excessive Somnolence; Intermittent Claudication; Spinal Cord Injuries; Fetal Membranes, Premature Rupture; Consciousness; Sleepiness | Details | |||||||
| Regadenoson Hydrate | CVT-3146 | Approved | Astellas Pharma Inc | Lexiscan, Rapiscan | United States | Contrast agents | Astellas Pharma Inc | 2008-04-10 | Myocardial Ischemia; Rejection of lung transplantation; Cardiovascular contrast agents; Pneumonia; Coronary Artery Disease; Heart Diseases; Glioblastoma; Coronavirus Disease 2019 (COVID-19); Retinal Artery Occlusion; Oligodendroglioma; Contrast agents; Cardiovascular Diseases; Astrocytoma; Diagnostic agents; Glioma; Anemia, Sickle Cell | Details |
| Caffeine/Ergotamine Tartrate | Approved | Novartis Pharma Ag | Cafergot | Mainland China | Migraine Disorders | Tianjin Medical University Pharmaceutical Factory | 1948-11-26 | Migraine Disorders | Details | |
| Oxtriphylline | Approved | Theocolin | Canada | null | Asthma | Details | ||||
| Caffeine/Metamizole Sodium/Dihydroergotamine Mesylate | Approved | Cefaliv | Migraine Disorders | Details | ||||||
| Adenine | Approved | Leucon | Japan | Leukopenia | Ohara Pharmaceutical Co Ltd | Leukemia; Leukopenia | Details | |||
| Istradefylline | KW-6002 | Approved | Kyowa Hakko Kirin Co Ltd | Nourianz, Nouriast | Japan | Parkinson Disease | Kyowa Hakko Kirin Co Ltd | 2013-03-25 | Substance-Related Disorders; Restless Legs Syndrome; Sleep Wake Disorders; Parkinson Disease; Cognitive Dysfunction; Hepatic Insufficiency; Movement Disorders; Amyotrophic Lateral Sclerosis | Details |
| Acetaminophen/Methylephedrine Hydrochloride/Dextromethorphan Hydrobromide/Chlorphenamine Maleate/Caffeine/Sulfogaiacol | Approved | Mainland China | Common Cold | Zhejiang Apeloa Kangyu Pharmaceutical Co Ltd | Common Cold | Details | ||||
| Dextromethorphan Hydrobromide/Chlorphenamine Maleate/Sodium Citrate/Ammonium Chloride/Caffeine | Approved | 尔可芬 | Cough | Details | ||||||
| Acetaminophen/Caffeine/Codeine Phosphate/Diphenhydramine Hydrochloride | Approved | 斯力帮 | Headache | Details | ||||||
| Ferric Ammonium Citrate/Vitamin B1/Sodium glycerophosphate/Caffeine | Approved | Ischemia | Details | |||||||
| Norethidranum Dipropionas/Caffeine/Vitamin B6 | Approved | Mainland China | Contraception | Huazhong Pharmaceutical Co Ltd | 1981-01-01 | Contraception | Details | |||
| Acetaminophen/Propyphenazone/Caffeine | Approved | 散利痛, 散列通 | Common Cold | Details | ||||||
| Quinine Hydrochloride Hydrate/Caffeine | Approved | Malaria | Details | |||||||
| Dihydroergocryptine/Caffeine | Approved | 洛斯宝, VASOBRAL | Diabetic Retinopathy; Diabetes Mellitus | Details | ||||||
| Aminophenazone/Phenacetin/Caffeine | Approved | Pain; Fever | Details | |||||||
| Aminophenazone/Caffeine/Calcium Glycerophosphate | Approved | Headache; Dysmenorrhea; Neuralgia | Details | |||||||
| Acetaminophen/Caffeine/Chlorphenamine Maleate | Approved | Common Cold | Details | |||||||
| Aminophenazone/Caffeine/Chlorphenamine Maleate | Approved | Headache; Common Cold; Neuralgia | Details | |||||||
| Aspirin/Caffeine/Codeine Phosphate | Approved | 柯克 | Pain | Details | ||||||
| Acetaminophen/Pseudoephedrine Hydrochloride/Caffeine | Approved | Common Cold | Details | |||||||
| Aspirin/Acetaminophen/Caffeine/Chlorphenamine Maleate | Approved | Common Cold | Details | |||||||
| Aspirin/Caffeine/Chlorphenamine Maleate/Tartaric Acid | Approved | Common Cold | Details | |||||||
| Acetaminophen/Caffeine/Pseudoephedrine Hydrochloride/Chlorphenamine Maleate | Approved | Common Cold | Details | |||||||
| Aminophenazone/Phenacetin/Caffeine/Phenobarbital | Approved | Migraine Disorders | Details | |||||||
| Acetaminophen/Chlorphenamine Maleate/Caffeine/Guaifenesin | Approved | Common Cold | Details | |||||||
| Acetaminophen/Amantadine Hydrochloride/Caffeine/Artificial Bezoar | Approved | Common Cold | Details | |||||||
| Acetaminophen/Salicylamide/Pseudoephedrine Hydrochloride/Caffeine/Triprolidine Hydrochloride Hydrate | Approved | 联邦菲迪乐 | Common Cold | Details | ||||||
| Sodium Benzoate/Caffeine | Approved | Central Nervous System Diseases | Details | |||||||
| Aminophenazone/Caffeine | Approved | Fever | Details | |||||||
| Antipyrine/Caffeine | Approved | Common Cold | Details | |||||||
| Aspirin/Caffeine | Approved | Common Cold | Details | |||||||
| Antipyrine/Caffeine/Citric Acid | Approved | 米格来宁 | Mainland China | Sedation | Xi'An Disai Biological Pharmaceutical Co Ltd, Heilongjiang Hasing Pharmaceutical Group Co Ltd, Huadong Medicine (Xi'An) Bodyguard Pharmaceutical Co Ltd, Mudanjiang Lingtai Medicdment Co Ltd | 1982-01-01 | Sedation | Details | ||
| Aminophenazone/Acetaminophen/Caffeine/Chlorphenamine Maleate | Approved | 克感敏, 扑感敏, 朴感敏 | Common Cold | Details | ||||||
| Acetaminophen/Caffeine/Ephedrine Hydrochloride/Diphenhydramine Hydrochloride | Approved | Influenza, Human | Details | |||||||
| Acetaminophen/Caffeine/Chlorphenamine Maleate/Artificial Bezoar | Approved | Common Cold | Details | |||||||
| Aspirin/Phenacetin/Caffeine | Approved | Headache; Dysmenorrhea; Toothache; Neuralgia; Fever; Arthralgia | Details | |||||||
| Aspirin/Caffeine/Acetaminophen | Approved | United States | Migraine Disorders | Haleon PLC | Migraine Disorders; Anesthesia | Details | ||||
| Acetaminophen/Butalbital/Caffeine | Approved | Headache | Details | |||||||
| Acetaminophen/Caffeine | Approved | Zentiva As | Mainland China | Pain | Sino--American Shanghai Squibb Pharmaceutical Ltd | Pain | Details | |||
| Sodium glycerophosphate/Caffeine/Vitamin B/Phosphoric Acid/Niacin | Approved | Details | ||||||||
| Acetaminophen/Caffeine/Phenylephrine Hydrochloride/Chlorphenamine Maleate/Vitamin B | Approved | Details | ||||||||
| Acetaminophen/Chlorphenamine Maleate/Methylephedrine Hydrochloride/Sulfogaiacol/Caffeine | Approved | Mainland China | Common Cold | Hainan Sanfengyou Pharmaceutical Co Ltd | 2006-05-16 | Common Cold | Details | |||
| Brompheniramine Maleate/Bromhexine Hydrochloride/Caffeine/Acetaminophen/Phenylephrine Hydrochloride | Approved | Common Cold | Details | |||||||
| Acetaminophen/Amantadine Hydrochloride/Caffeine/Chlorphenamine Maleate/Artificial Bezoar | Approved | 复方氨酚烷胺 | Mainland China | Common Cold | null | 1984-01-01 | Common Cold | Details | ||
| Caffeine | Approved | Fatigue; Mental Fatigue; Memory Disorders; Cocaine-Related Disorders; Sexual Dysfunction, Physiological; Parkinson Disease; Cognitive Dysfunction; Diabetes Complications; Diabetic Nephropathies; Postoperative Cognitive Complications; Diabetes Mellitus, Type 1; Delirium; Apnea; Disorders of Excessive Somnolence; Intermittent Claudication; Spinal Cord Injuries; Fetal Membranes, Premature Rupture; Consciousness; Sleepiness | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Naproxen/Caffeine | BAY-2880376 | Phase 3 Clinical | Bayer AG | Pain, Postoperative | Details |
| Caffeinol | Phase 3 Clinical | Cardium Therapeutics | Alcoholic Intoxication; Stroke; Alcoholism; Disruptive, Impulse Control, and Conduct Disorders; Carcinoma, Non-Small-Cell Lung | Details | |
| Imaradenant | AZD-4635; HTL-1071 | Phase 2 Clinical | Nxera Pharma UK Ltd | Solid tumours; Prostatic Neoplasms, Castration-Resistant | Details |
| Ciforadenant | CPI-444; V-81444 | Phase 2 Clinical | Vernalis (R&D) Ltd | Carcinoma, Renal Cell; Neoplasms; Attention Deficit Disorder with Hyperactivity; Multiple Myeloma; Parkinson Disease; Carcinoma, Non-Small-Cell Lung | Details |
| Etrumadenant | AB-928; GS-0928 | Phase 2 Clinical | Arcus Biosciences Inc | Prostatic Neoplasms; Melanoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Pancreatic Ductal; Endometrial Neoplasms; Laryngeal Neoplasms; Mouth Neoplasms; Pharyngeal Neoplasms; Carcinoma, Squamous Cell; Lung Neoplasms; Colorectal Neoplasms; Oropharyngeal Neoplasms; Breast Neoplasms; Ovarian Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Hypopharyngeal Neoplasms; Small Cell Lung Carcinoma; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma, Merkel Cell; Rectal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Head and Neck Neoplasms | Details |
| KW-6356 | KW-6356 | Phase 2 Clinical | Kyowa Hakko Kirin Co Ltd | Parkinson Disease; Hepatic Insufficiency | Details |
| PORT-8 | PORT-8; TT-53 | Phase 2 Clinical | Tarus Therapeutics Inc | Solid tumours | Details |
| PORT-6 | PORT-6; TT-10 | Phase 2 Clinical | Impetis Biosciences Ltd | Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| VG081821AC | Phase 2 Clinical | Zhejiang Vimgreen Pharmaceuticals Ltd | Parkinson Disease | Details | |
| ILB-2109 | WTS-001; ILB-2109 | Phase 2 Clinical | Hangzhou Wutong Tree Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| PBF-999 | PBF-999 | Phase 1 Clinical | Palobiofarma Sl | Neoplasms; Huntington Disease | Details |
| SHR-5126 | SHR-5126 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Neoplasms | Details |
| Taminadenant | NIR-178; PBF-509 | Phase 1 Clinical | Palobiofarma Sl | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Carcinoma, Renal Cell; Carcinoma; Triple Negative Breast Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Colorectal Neoplasms; Parkinson Disease; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| ST-4206 | ST-4206 | Phase 1 Clinical | Alfasigma Spa | Parkinson Disease | Details |
| JNJ-86974680 | JNJ-86974680 | Phase 1 Clinical | Johnson & Johnson Enterprise Innovation Inc | Carcinoma, Non-Small-Cell Lung | Details |
| YZJ-5053 | YZJ5053; YZJ-5053 | Phase 1 Clinical | Shanghai Haiyan Pharmaceutical Technology Co Ltd | Solid tumours; Neoplasm Metastasis | Details |
| DZD-2269 | Phase 1 Clinical | Dizal Pharmaceutical Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| INCB-106385 | INCB-106385 | Phase 1 Clinical | Incyte Corp | Solid tumours; Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Triple Negative Breast Neoplasms; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular | Details |
| CS-3005 | CS-3005 | Phase 1 Clinical | Cstone Pharmaceuticals (Suzhou) Co Ltd | Solid tumours | Details |
| Sodium Citrate/Citric Acid/Dextrose/Monosodium phosphate/Adenine/Mannitol/Sodium Chloride | Details | ||||
| Sodium Citrate/Citric Acid/Dextrose | Details | ||||
| Sodium Citrate/Citric Acid/Dextrose/Monosodium phosphate/Adenine | Details | ||||
| Aspirin/Acetaminophen/Caffeine/Thiamine | Details | ||||
| Propranolol hydrochloride/Caffeine/Phenytoin Sodium | Details | ||||
| Ethenzamide/Caffeine/Chlorzoxazone | Details | ||||
| Salicylamide/Acetaminophen/Caffeine | Details | ||||
| Naproxen/Caffeine | BAY-2880376 | Phase 3 Clinical | Bayer AG | Pain, Postoperative | Details |
| Caffeinol | Phase 3 Clinical | Cardium Therapeutics | Alcoholic Intoxication; Stroke; Alcoholism; Disruptive, Impulse Control, and Conduct Disorders; Carcinoma, Non-Small-Cell Lung | Details | |
| Imaradenant | AZD-4635; HTL-1071 | Phase 2 Clinical | Nxera Pharma UK Ltd | Solid tumours; Prostatic Neoplasms, Castration-Resistant | Details |
| Ciforadenant | CPI-444; V-81444 | Phase 2 Clinical | Vernalis (R&D) Ltd | Carcinoma, Renal Cell; Neoplasms; Attention Deficit Disorder with Hyperactivity; Multiple Myeloma; Parkinson Disease; Carcinoma, Non-Small-Cell Lung | Details |
| Etrumadenant | AB-928; GS-0928 | Phase 2 Clinical | Arcus Biosciences Inc | Prostatic Neoplasms; Melanoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Pancreatic Ductal; Endometrial Neoplasms; Laryngeal Neoplasms; Mouth Neoplasms; Pharyngeal Neoplasms; Carcinoma, Squamous Cell; Lung Neoplasms; Colorectal Neoplasms; Oropharyngeal Neoplasms; Breast Neoplasms; Ovarian Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Hypopharyngeal Neoplasms; Small Cell Lung Carcinoma; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma, Merkel Cell; Rectal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Head and Neck Neoplasms | Details |
| KW-6356 | KW-6356 | Phase 2 Clinical | Kyowa Hakko Kirin Co Ltd | Parkinson Disease; Hepatic Insufficiency | Details |
| PORT-8 | PORT-8; TT-53 | Phase 2 Clinical | Tarus Therapeutics Inc | Solid tumours | Details |
| PORT-6 | PORT-6; TT-10 | Phase 2 Clinical | Impetis Biosciences Ltd | Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| VG081821AC | Phase 2 Clinical | Zhejiang Vimgreen Pharmaceuticals Ltd | Parkinson Disease | Details | |
| ILB-2109 | WTS-001; ILB-2109 | Phase 2 Clinical | Hangzhou Wutong Tree Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| PBF-999 | PBF-999 | Phase 1 Clinical | Palobiofarma Sl | Neoplasms; Huntington Disease | Details |
| SHR-5126 | SHR-5126 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Neoplasms | Details |
| Taminadenant | NIR-178; PBF-509 | Phase 1 Clinical | Palobiofarma Sl | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Carcinoma, Renal Cell; Carcinoma; Triple Negative Breast Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Colorectal Neoplasms; Parkinson Disease; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| ST-4206 | ST-4206 | Phase 1 Clinical | Alfasigma Spa | Parkinson Disease | Details |
| JNJ-86974680 | JNJ-86974680 | Phase 1 Clinical | Johnson & Johnson Enterprise Innovation Inc | Carcinoma, Non-Small-Cell Lung | Details |
| YZJ-5053 | YZJ5053; YZJ-5053 | Phase 1 Clinical | Shanghai Haiyan Pharmaceutical Technology Co Ltd | Solid tumours; Neoplasm Metastasis | Details |
| DZD-2269 | Phase 1 Clinical | Dizal Pharmaceutical Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| INCB-106385 | INCB-106385 | Phase 1 Clinical | Incyte Corp | Solid tumours; Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Triple Negative Breast Neoplasms; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular | Details |
| CS-3005 | CS-3005 | Phase 1 Clinical | Cstone Pharmaceuticals (Suzhou) Co Ltd | Solid tumours | Details |
| Sodium Citrate/Citric Acid/Dextrose/Monosodium phosphate/Adenine/Mannitol/Sodium Chloride | Details | ||||
| Sodium Citrate/Citric Acid/Dextrose | Details | ||||
| Sodium Citrate/Citric Acid/Dextrose/Monosodium phosphate/Adenine | Details | ||||
| Aspirin/Acetaminophen/Caffeine/Thiamine | Details | ||||
| Propranolol hydrochloride/Caffeine/Phenytoin Sodium | Details | ||||
| Ethenzamide/Caffeine/Chlorzoxazone | Details | ||||
| Salicylamide/Acetaminophen/Caffeine | Details |
This web search service is supported by Google Inc.
