
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Microelectrode Array (MEA) Service Technology
Platforms
We offer industry-leading Microelectrode Array (MEA) service for electrophysiological activity detection in 2D cells and organoids. Our system enables real-time, non-invasive recording of spontaneous and stimulated electrical signals under stable conditions, providing comprehensive data and reports for advanced research on neuronal and cardiac networks.
MEA is an advanced electrophysiological detection technology that uses multiple microelectrodes embedded on the surface of culture plates to simultaneously monitor and record electrical signals from multiple cells. These electrodes are in close contact with the cells, allowing electrical signals generated by the cells to be transmitted through the cell-electrode interface and captured by the MEA system as analyzable data. With its efficiency, non-invasiveness, and ability for long-term continuous monitoring, MEA technology is a powerful tool for research in neuroscience, developmental biology, and drug development.
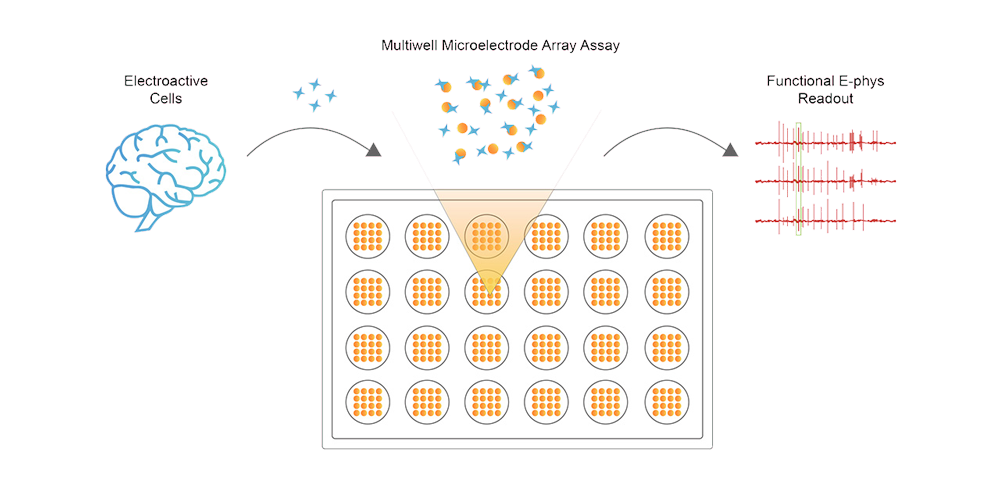
Microelectrode Array (MEA) Technology
What You Can Do with ACRO's MEA System

Case Study
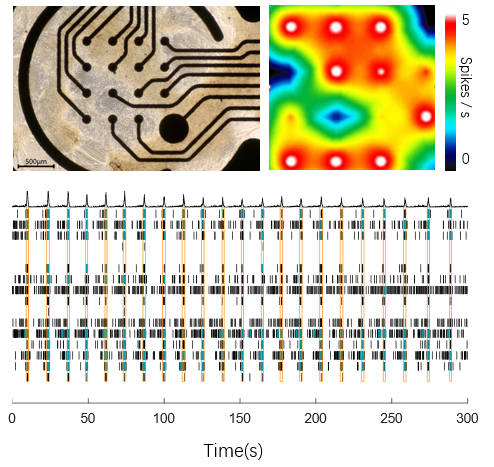
This data set highlights the intrinsic activity of our Human iPSC-Derived Dopamine Neurons (Cat. No. CIPC-DDC001) cultured on an MEA plate. The neurons exhibit robust spontaneous firing, as visualized in the accompanying heatmap video. The raster plot clearly shows regular network burst firing patterns, indicative of well-coordinated neuronal activity. The photo of the neurons on the MEA plate further confirms the successful culture and network formation, making this data a powerful demonstration of their functional connectivity and suitability for neurophysiological studies.
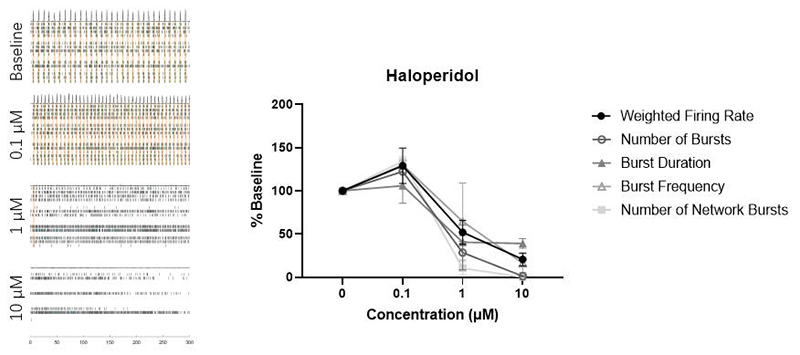
This data set demonstrates the dose-dependent effects of Haloperidol on neuronal firing. Raster plots and associated firing parameters (for Weighted Mean Firing Rate, Number of Bursts, Burst Duration, Burst Frequency, and Number of Network Bursts, n = 2) are presented for varying Haloperidol concentrations. At 0.1 μM, the firing activity is enhanced, while at 1 μM, the activity diminishes. At 10 μM, the firing is nearly abolished. These results are consistent with findings from Yokoi et al. (2019), where Haloperidol is known to inhibit D2 receptors at low doses and 5-HT2 receptors at high doses (Tyler et al., 2017). This confirms that the relevant receptors in our Human iPSC-Derived Dopamine Neurons (Cat. No. CIPC-DDC001) are functioning normally, underscoring their utility in drug screening and neurotoxicity studies.
This web search service is supported by Google Inc.









