 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > Ensuring the Safety and Efficacy of Biopharmaceuticals with resDetect™ Residual Detection Solutions The biopharmaceutical industry stands at the forefront of medical innovation, with Cell and Gene Therapies (CGTs) and therapeutic antibodies offering promising treatment options for a wide range of diseases. However, these groundbreaking therapies come with their own set of challenges, particularly in ensuring the purity and safety of the final products. One of the most critical issues facing manufacturers is the presence of residual impurities, which demands rigorous attention and advanced solutions.
Throughout the Chemistry, Manufacturing, and Controls (CMC) production phase, various materials are essential for creating these complex biotherapeutics. These include host cell components, plasmid DNA, enzymes, cytokines, antibodies, and antibiotics. While crucial for production, these elements can persist as impurities in the final product, potentially compromising safety and efficacy. Stringent regulations necessitate comprehensive testing and clearance of these process-related residuals to ensure both quality and regulatory compliance. This requirement has led to the development of advanced detection methods, such as ACROBiosystems’ resDetect™, which offers solutions for various types of impurities.
In the production of CGTs, expression systems like HEK293/HEK293T cells and E. coli are widely used for viral and plasmid vector production. However, residual host cell DNA (resDNA) from these systems can persist, posing potential safety concerns including carcinogenicity and the presence of infectious agents.
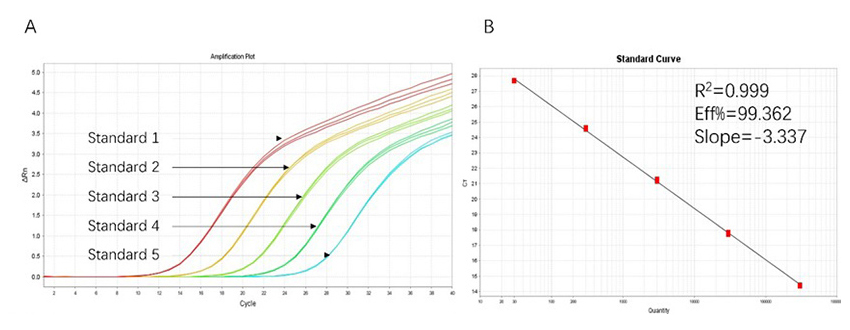
Figure 1. High sensitivity and broad dynamic range using the HEK293 resDNA Quantitation Kit. (A) Typical analysis results obtained with Standard 1 (300 pg/μL) to 5 (30 fg/μL). (B) The standard curve of the 10-fold dilution series. PCR efficiency should be 90-110%.
Similarly, Chinese Hamster Ovary (CHO) cells, crucial in therapeutic antibody production, can release DNA fragments during manufacturing. These fragments pose similar risks and must be carefully monitored and minimized to ensure the safety of products such as monoclonal antibodies, bispecific antibodies, and antibody-drug conjugates.
The Pichia pastoris expression system has become a popular tool in recombinant protein production, offering advantages of both prokaryotic and eukaryotic systems. This methylotrophic yeast provides cost-effective, high-level protein expression with eukaryotic post-translational modifications, making it suitable for producing various biopharmaceuticals. Its utility is demonstrated by FDA approvals of products like Caplacizumab and Eptinezumab. The system's ability to achieve high-density fermentation and efficient protein expression has made it valuable in biotechnology, enabling the production of diverse therapeutic proteins including growth hormones, human albumin, and collagen.
To address these challenges, resDetect™ offers a comprehensive range of highly sensitive residual host cell nucleic acid detection kits across various host cell types, providing robust support for resDNA quality control needs.
Plasmid DNA is at the core of many gene therapy strategies, serving as a crucial vector for gene delivery and expression. However, the potential presence of residual plasmid DNA in final therapeutic products presents both safety concerns and regulatory challenges. Residual plasmid DNA can lead to unintended gene expression, immunogenicity, insertional mutagenesis, and horizontal gene transfer of antibiotic resistance genes. These risks necessitate rigorous quantification and monitoring of residual plasmid DNA throughout the production process and in the final product.
Quantitative PCR (qPCR) has emerged as the gold standard for detection due to its high sensitivity and specificity. The qPCR-based detection kits offer detection limits as low as 1-10 copies, wide dynamic ranges, and multiplexing capabilities for simultaneous detection of multiple targets. Regulatory bodies, including the FDA and EMA, have established stringent guidelines for residual DNA levels in gene therapy products, typically enforcing a general threshold of 10 ng per dose. The resDetect™ Plasmid DNA Residual Detection Kits offer comprehensive monitoring capabilities throughout the production process and compatibility with various gene therapy products, including those utilizing viral vectors.
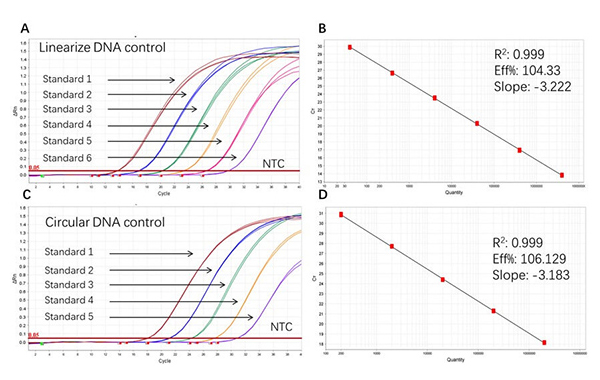
Figure 2. High sensitivity and broad dynamic range using the Plasmid resDNA Quantitation Kit. (A) Typical analysis results of obtained with Linearize DNA control Standard 1 (4×10⁶ copies/µL) to 6 (4×10 copies/µL). (B) The standard curve of the Linearize DNA control 10-fold dilution series. PCR efficiency should be 90-110%. (C) Typical analysis results of obtained with Circular DNA control Standard 1 (2×106 copies/µL) to 5 (2×10² copies/µL). (D) The standard curve of the Circular DNA control 10-fold dilution series. PCR efficiency should be 90-110%.
In the production of viral vectors for gene therapies, cell lines like HEK293 and HEK293T are widely used due to their efficiency in producing high-titer viral vectors. However, these cell lines contain oncogenes and viral sequences that can persist in the final therapeutic products, posing significant safety risks. The detection of viral oncogene residues is therefore crucial in ensuring the safety and efficacy of gene therapy products. These residues, if present, can lead to oncogenicity, genomic instability, and regulatory non-compliance.
To address these concerns, a variety of detection methodologies have been developed. Quantitative PCR (qPCR) based platforms, such as the resDetect™ kits, offer highly sensitive and specific detection of E1A and SV40LTA DNA residues in CGT production. These kits boast a quantitative detection limit of 40 copies/μL and can complete the experimental protocol within 4 hours, enabling efficient quality control. Regulatory bodies, including the FDA, have established stringent guidelines for the detection and control of residual viral oncogenes in gene therapy products. Manufacturers must validate their detection methods and adhere to specific safety testing and compliance standards to ensure that residual viral oncogenes do not exceed acceptable limits, thereby mitigating oncogenic risks in the final therapeutic products.
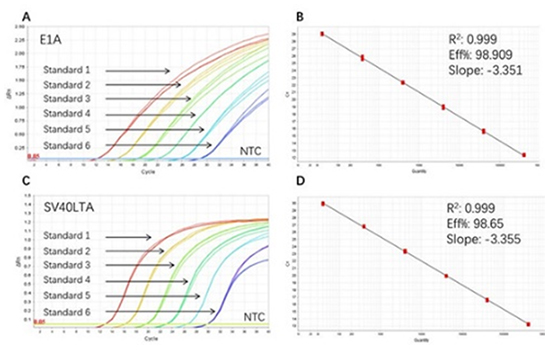
Figure 3. High sensitivity and broad dynamic range using the E1A&SV40LTA resDNA Quantitative Kit. (A) Typical analysis results of obtained with E1A DNA Control Standard 1 (4×10⁶ copies/µL) to 6 (4×10 copies/µL). (B) The standard curve of the E1A DNA Control 10-fold dilution series. PCR efficiency should be 90-110%. (C) Typical analysis results of obtained with SV40LTA DNA Control Standard 1 (4×10⁶ copies/µL) to 6 (4×10 copies/µL). (D) The standard curve of the SV40LTA DNA controls 10-fold dilution series. PCR efficiency should be 90-110%.
The detection and quality control of residual enzymes such as nucleases (DNases and RNases) and CRISPR/Cas proteins have emerged as crucial quality control measures in the rapidly evolving field of CGT. These enzymes, while essential in various stages of CGT production, can pose significant risks if present in the final product. Residual nucleases can degrade therapeutic nucleic acids, compromising the integrity of gene therapies, while CRISPR/Cas proteins may cause off-target genetic modifications. Additionally, these enzymes have the potential to trigger unwanted immune responses in patients.
To address these challenges, quantitative PCR (qPCR) based platforms, such as the resDetect™ kits, offer highly sensitive and specific detection of residual enzymes. These kits provide rapid protocols, typically completed within a few hours, enabling efficient quality control throughout the CGT production process. The high sensitivity of these assays ensures compliance with stringent regulatory requirements, while their specificity allows for tailored detection of specific enzymes like DNases, RNases, and Cas proteins.
Regulatory agencies, including the FDA and EMA, have also established guidelines for the control and detection of process-related impurities, including enzyme residues. Manufacturers must establish and justify acceptable levels of residual enzymes, validate their removal and detection processes, and provide comprehensive documentation of their control strategies. These measures are essential not only for ensuring product safety and efficacy but also for meeting regulatory requirements in the rapidly advancing field of cell and gene therapy.
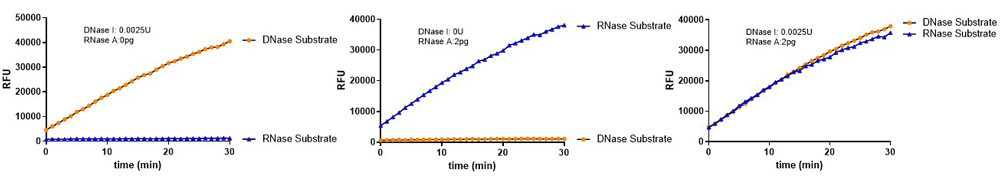
Figure 4. DNase Activity Assay Kit (Fluorescence) (Cat. No. ASE-A002) and RNase Activity Assay Kit (Fluorescence) (Cat. No. ASE-A001) were used to detect nuclease residues in the same sample. No significant cross-reactivity or interference was observed.
In the rapidly advancing field of immune cell therapies, the detection and control of residual cytokines and antibodies used during manufacturing have become critical quality control measures. These molecules, such as interleukins (IL-2, IL-15, IL-7) and anti-CD3/CD28 antibodies, are essential for cell proliferation and differentiation during production. However, their presence in the final product can pose significant risks, including unwanted immune responses in patients, altered therapeutic efficacy, and regulatory non-compliance.
To address these challenges, advanced detection methodologies have been developed. These include highly specific ELISA tests, multiplex bead-based assays, and mass spectrometry techniques. Notably, the resDetect™ platform offers comprehensive assay kits designed specifically for cell therapy products, providing high sensitivity, specificity, and compatibility with complex biological matrices. These tools are crucial for ensuring precise detection during CMC process quality control, thereby facilitating regulatory compliance and product safety.
As the field evolves, emerging trends include the integration of real-time, in-line monitoring, development of multi-analyte assays, and increased automation of detection methods. By prioritizing rigorous cytokine and antibody residue detection, manufacturers can enhance their quality control processes, minimize risks associated with these impurities, and ultimately deliver safer and more effective cell therapy products to patients. This focus on residue detection is not just a regulatory requirement but a critical step in advancing the promise of immune cell therapies in treating various diseases.
The detection and control of antibiotic residues in cell and gene therapy (CGT) products are critical to ensuring their safety and efficacy. Antibiotics like kanamycin and gentamicin are commonly used in cell culture processes during CGT manufacturing. However, their presence in the final product can pose significant risks, including ototoxicity, nephrotoxicity, allergic reactions, and contributing to antimicrobial resistance. Regulatory bodies such as the FDA and EMA emphasize minimizing antibiotic residues, particularly β-lactam antibiotics like penicillin, due to the potential for severe allergic reactions.
Advanced detection kits, such as those offered by the resDetect™ platform, provide essential tools for manufacturers to ensure product safety, efficacy, and regulatory compliance. The resDetect™ platform offers highly sensitive and specific antibiotic residue detection kits designed for CGT manufacturing, capable of detecting low levels of residues to meet stringent regulatory requirements. These kits are user-friendly, require minimal sample volumes, and are applicable across various stages of CGT production workflows. As the industry evolves, integrating real-time monitoring, developing multi-analyte assays, adopting rapid testing methods, and harmonizing standards will be crucial to support the production of increasingly sophisticated cell and gene therapies.
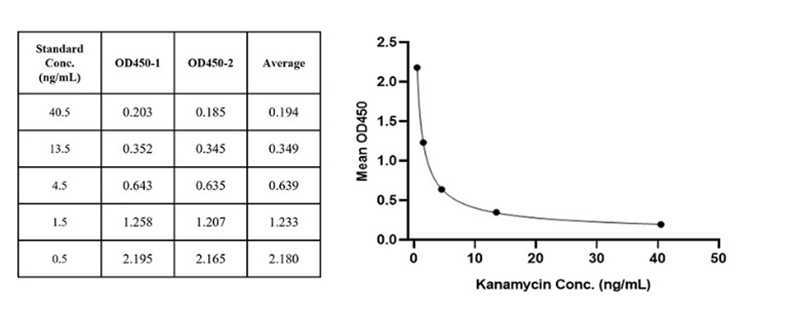
Figure 5. For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipment. The following example data is for reference only. (Cat. No. RES-A004)
Effective detection and control of residual impurities are crucial for ensuring the safety and efficacy of biopharmaceutical products. ACROBiosystems resDetect™ solutions offer dependable, sensitive, specific, and thoroughly validated tools for monitoring various types of residual impurities throughout the CMC manufacturing processes. By enabling precise monitoring and control of these impurities, resDetect™ kits can help safeguard patient well-being and foster confidence in the integrity of biopharmaceutical products.
> resDetect™ CGT CMC Manufacturing Process Residue Detection Solutions
This web search service is supported by Google Inc.
