
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
T lymphocytes play a crucial role in the human immune system, and their unique properties form the foundation of adoptive T cell therapies. These innovative approaches harness the inherent functions of specific T cells to combat a range of diseases. Among the most promising T cell-based therapies are Chimeric Antigen Receptor T cell (CAR-T) therapy and Tumor-Infiltrating Lymphocyte (TIL) therapy. These revolutionary techniques have demonstrated the ability to generate persistent and effective clinical responses in patients, offering a promising future in the treatment of previously intractable conditions.
Despite their potential, the widespread implementation of T cell therapies faces significant challenges, particularly in scaling up the Chemistry, Manufacturing, and Controls (CMC) process for engineering T cells. This complex procedure involves multiple intricate steps, including ex vivo activation, expansion, and genetic modification of T cells. As the field advances, optimizing and standardizing these processes is crucial to ensure the consistent production of high-quality, therapeutically effective T cells. Addressing these challenges is essential for realizing the full potential of T cell-based therapies and making them accessible to a broader patient population.
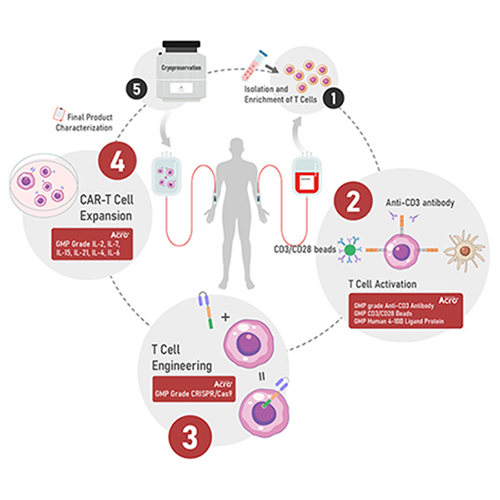
Figure 1. Manufacturing process of CAR-T Cell therapy workflow
ACROBiosystems is dedicated to supporting research in immune cell therapy. Leveraging our robust cell culture platform, antibody development platform, flow cytometry validation platform, and GMP quality management system, we have developed a series of materials and services for the CMC production process of T cells. Our offerings include solutions for T cell activation, T cell culture, T cell engineering, and CMC process purification. These solutions are designed to support scientists and pharmaceutical companies worldwide in advancing the development of adoptive T cell therapies.
• T cell Activation
The activation of T cells is a critical step in adoptive T cell therapies. This process typically employs anti-CD3 and anti-CD28 antibodies, or CD3/CD28 antibody-conjugated magnetic beads to mimic the signaling processes in vivo to activate T cells. Additionally, specific growth factors and cytokines are introduced to the culture medium, as they play a crucial role in stimulating T cell proliferation and differentiation in vitro.
High-quality, GMP-grade ancillary reagents are essential for this process. For instance, anti-CD3 (OKT3) antibody and CD3/CD28 antibody-conjugated magnetic beads that meet stringent quality standards are commercially available. These products undergo rigorous virus removal steps and testing, are free from animal-derived components, and offer high stability, batch-to-batch consistency, and enhanced safety profiles.
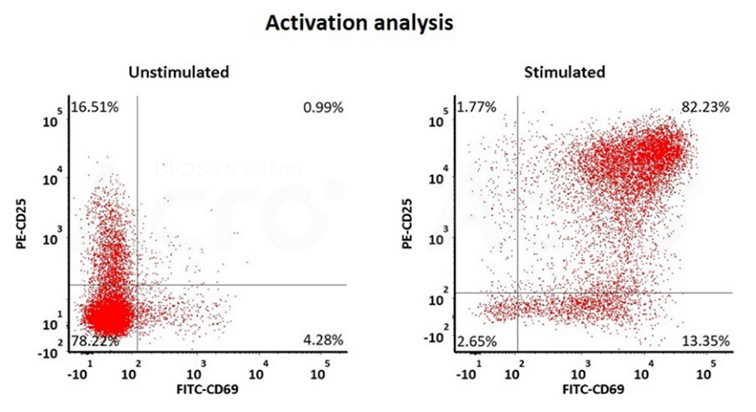
The human T cells were effectively stimulated with GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads (Cat. No. GMP-MBS001)
• T Cell Culture
Following initial activation through signal stimulation, T cell proliferation and further activation are dependent on a cocktail of cytokines. High-quality GMP-grade cytokines, including IL-2, IL-7, IL-15, IL-21, and TNF-α, have been developed specifically for T cell culture applications. These cytokines are produced under rigorous quality management systems and subjected to GMP-grade release testing, ensuring their suitability for advanced cell therapy applications.
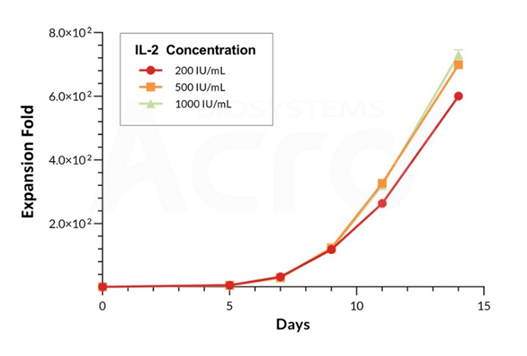
Cell viability curve of PBMCs cultured with IL-2. Increasing concentrations of GMP IL-2 (Cat. No. GMP-L02H14) were added as a growth factor supplement to PBMCs showing consistent and high cell viabilities up to Day 14.
• T cell Engineering
In allogeneic CAR-T therapy, the introduction of CRISPR/Cas gene-editing technology has emerged as a powerful tool to mitigate the risks of allogeneic rejection and graft-versus-host disease (GvHD). GMP-grade gene-editing tools, such as GENPower™ NLS-Cas9 Nuclease, have been engineered to exhibit high bioactivity and TCR knockout efficiency, as validated through both in vitro and in vivo studies. These tools, characterized by ultra-low endotoxin levels, are designed to streamline and accelerate T cell engineering processes.
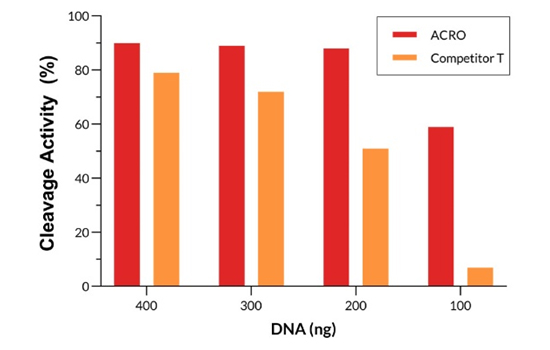
Different amounts of Cas9 were incubated with the same amount of excess gRNA and plasmid for 60 minutes at 37°C. When using 400-200 ng ACRO Cas9, the cutting efficiency is greater than 90%. In comparison, when using a 200 ng Competitor T, the cutting efficiency is only about 50%.
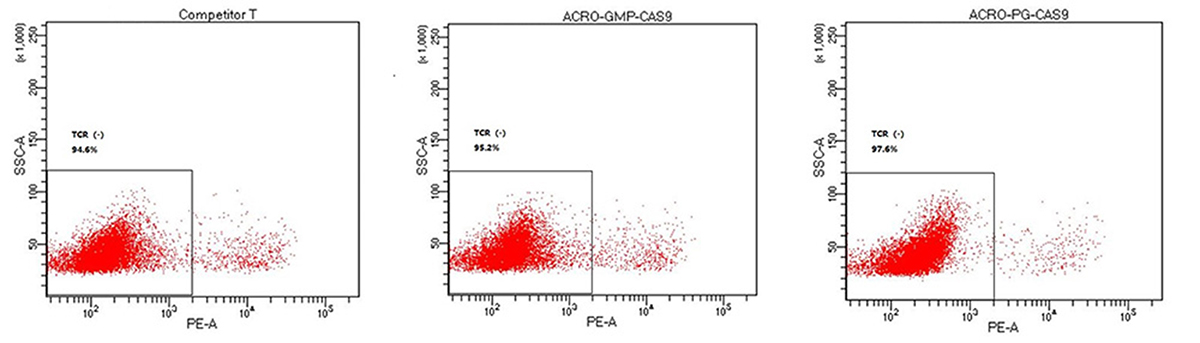
The gRNA and plasmid cutting efficiency and the TCR knockout efficiency with GMP GENPower™ NLS-Cas9 Nuclease (Cat. No. GMP-CA9S18)
• Process Purification
The production of lentiviral packaging plasmids, a critical step in CAR-T cell preparation, can result in nucleic acid residues that pose potential risks of infection or tumorigenicity ricks. Addressing this challenge is crucial for bioproduct safety regulation.
Leveraging advanced enzyme modification technology platforms, GMP-grade universal nucleases have been developed through AI simulation and high-throughput screening. These nucleases, available in conventional (GMP-NUES19) and salt active (GMP-NUES13) versions, are optimized for efficient removal of nucleic acid contamination in biopharmaceutical processes.
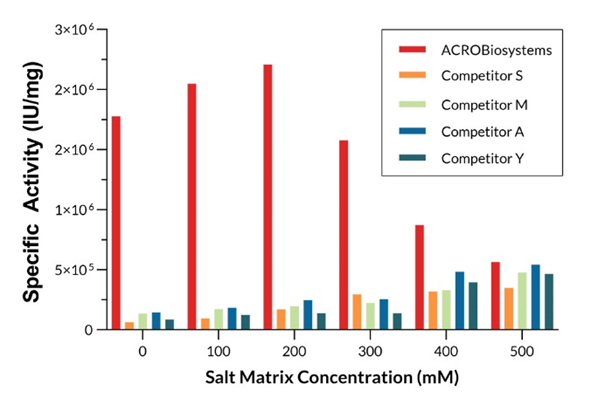
GMP Salt Active GENIUS ™ Nuclease (Cat. No. GMP-NUES13) can maintain high enzyme activity under 0-500mM NaCl conditions; The enzyme activity of GMP-NUES13 under 0-500mM conditions is significantly better than that of competitors.
This web search service is supported by Google Inc.







