
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > SARS-CoV-2 Infection: Do You Know About the Antibody-Dependent Enhancement (ADE)? As the COVID-19 resurgence is threatening people’s lives and health, recent developments of vaccines are providing a needed hope of controlling and arresting the pandemic. The tireless efforts of scientists, multiple labs, and pharmaceutical companies are bringing both traditional and newer technology based vaccines through the clinical pipelines in record pace.
On Nov. 9, Pfizer/BioNTech released their Phase III clinical trial data. Their mRNA vaccine has an effective rate of about 95%, with no severe side effects reported. They are the first to release the positive Phase III trial result, which is considered a big milestone. Then a week later, on Nov. 16, Moderna released a preliminary analysis of the 30,000-volunteer study, in which the vaccine was 94.5% effective at preventing Covid-19 based on 95 cases of symptomatic infection, 90 of them in the placebo group.
Safety and effectiveness of the vaccines are always the two main focus in any vaccine development. Antibody drug enhancement (ADE) risk poses a safety concern that must be mitigated during the clinical evaluation of these vaccines.
ADE can increase the severity of multiple viral infections and has been documented to occur through two distinct mechanisms in viral infections. It increases viral infection and replication by enhanced antibody-mediated virus uptake into Fc gamma receptor IIa (FcγRIIa)-expressing phagocytic cells or causes enhanced inflammation and immunopathology by excessive antibody Fc-mediated effector functions or immune complex formation.
ADE phenomenon was first described with the arbovirus by Hawkes in 1964. In 1977, Scott Halstead first identified ADE, where the antibodies generated from a first dengue infection can sometimes worsen the symptoms from a second infection. ADE has been observed in SARS, MERS as well as some other human respiratory virus (Fig. 1)
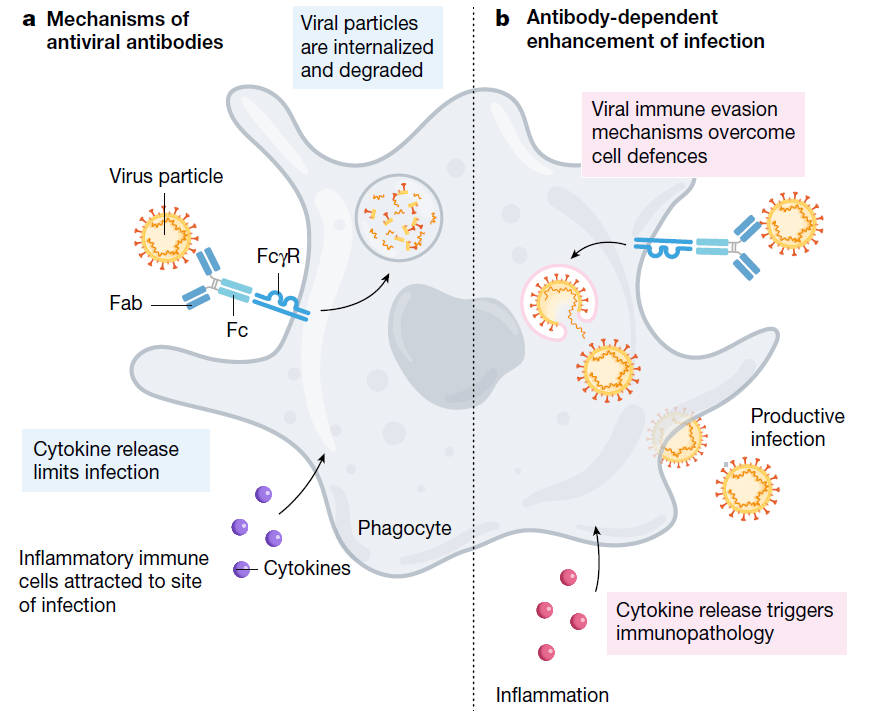
Fig. 1 Mechanism of ADE
The mechanism of ADE was not fully explained. But with the knowledge of ADE, scientists could determine the ADE related antigen and modify the lead molecules to make it safer and more effective.
So far, there was no ADE reported during the pre-clinical/clinical development of the COVID-19 vaccine. ADE related research still needs to be carried out to avoid severe side effects.
ACROBiosystems has developed a series of products, which can be used for the measurement of the anti-SARS-CoV-2 antibody isotypes like IgG1, IgG2, IgG3, and IgG4.
Product list
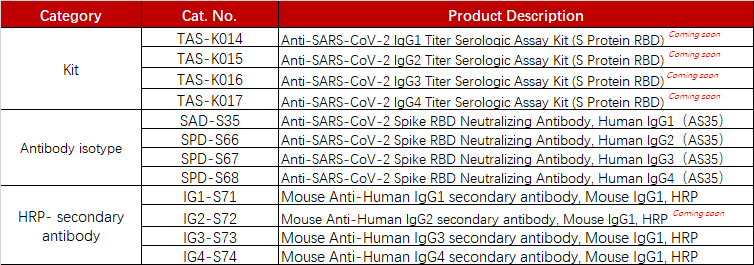
To support the vaccine development and evaluation, ACROBiosystems developed various COVID recombinant proteins and kits including Spike trimer proteins and antibody titer assay kits for IgG/IgM, the neutralizing antibody, and the antigen.
What’s more, the ACRO neutralizing antibody titer assay kit and RBD/Spike antibody titer assay kit are CE certified. These kits are intended for diagnostic and vaccine evaluation, with features such as convenient, fast, accurate, high throughput, high signal to noise ratio, etc.
Assay Data
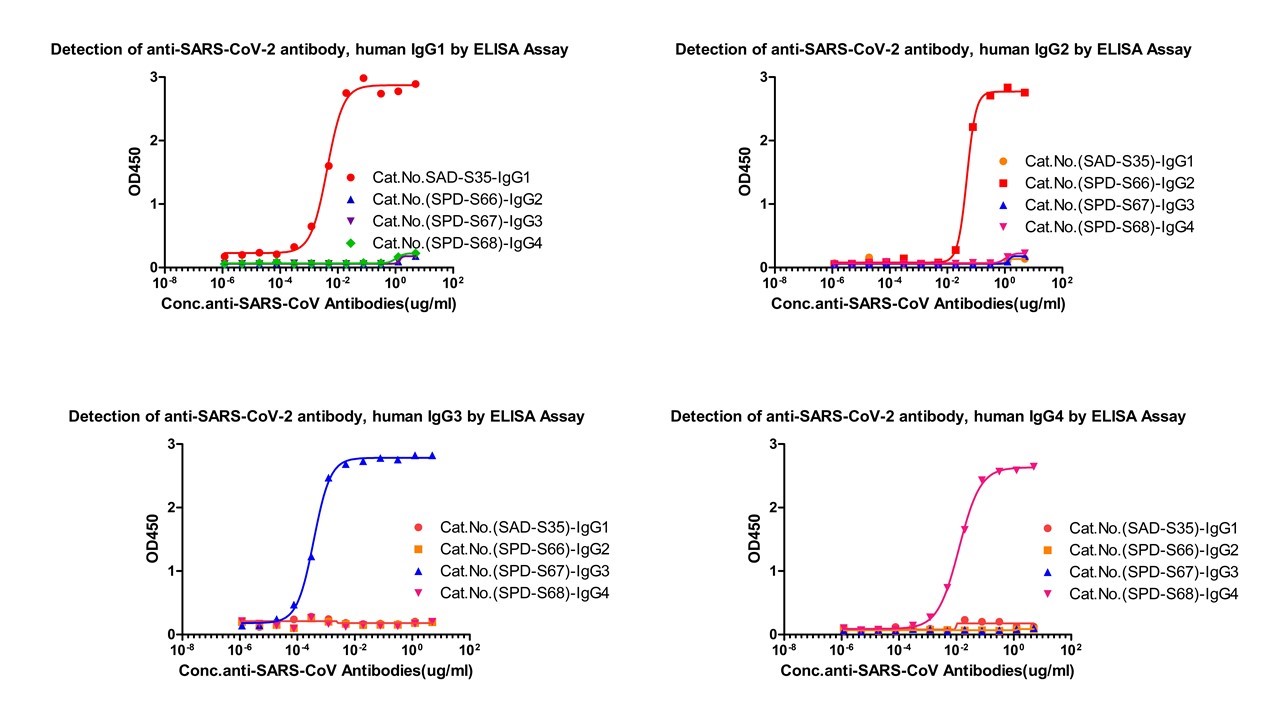
Fig.1 Cross-validation results of four IgG antibody subtypes detection
This web search service is supported by Google Inc.







