
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order Happy Holiday! Limited Keychain here with your next order
Happy Holiday! Limited Keychain here with your next order
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > Utilizing GMP-Grade Laminin for Scalable Manufacturing of iPSC-Derived Cell Therapies 
Induced pluripotent stem cells (iPSCs) represent a groundbreaking advancement in cell therapy and regenerative medicine. Their unique ability to differentiate into virtually any cell type within the human body offers promising prospects for developing innovative allogeneic cell therapies. These therapies could potentially address a wide range of diseases, including cardiovascular, neurological, ophthalmological, cancer, and metabolic disorders. Additionally, engineered iPSC-derived immunotherapy has emerged as a promising avenue for treating cancer and autoimmune diseases.
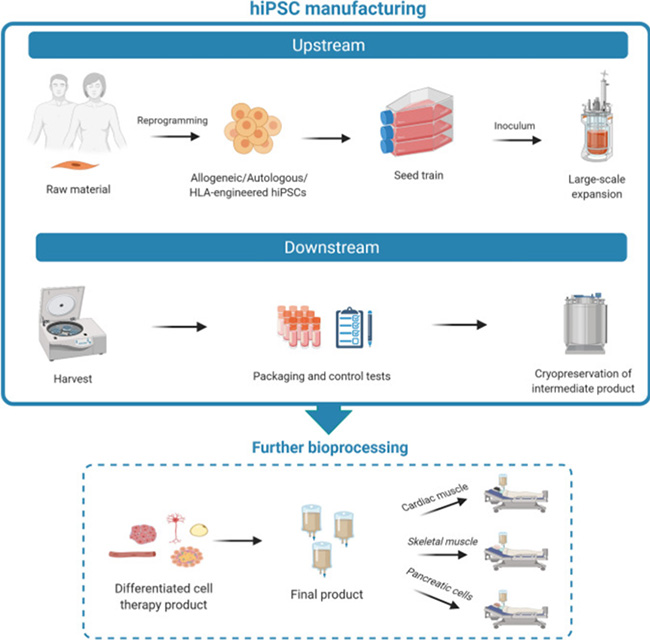
Fig. 1 illustrates the manufacturing process of iPSCs encompassing both upstream and downstream phases.
Additionally, subsequent bioprocessing steps are depicted, crucial for the refinement and generation of various functional, differentiated cell types.
However, the clinical application of iPSC technology faces notable challenges due to the delicate nature of these cells and their dependence on a supportive extracellular matrix environment for efficient expansion. The manufacturing process of iPSCs involves both upstream expansion and downstream differentiation phases, followed by further bioprocessing to generate functional, differentiated cell products (Figure 1). Whereas differentiation of iPSCs is well defined, scalable upstream iPSCs expansion under the required regulatory guidance for clinical use remains a challenge.
A critical determinant of iPSC quality and yield during expansion is the extracellular matrix used to facilitate cell adhesion and proliferation. Over the past decade, substantial advancements have been made in optimizing specialized culture media and matrices tailored specifically for iPSC culture. To achieve scalable, clinical-grade production of iPSCs from biological donors, an ideal matrix should serve as an effective yet consistent scaffold for cell adhesion and proliferation while preserving pluripotency.
Laminin, a large adhesive glycoprotein composed of heterotrimers of α, β, and γ chains, has emerged as a pivotal protein in the extracellular matrix that supports the undifferentiated expansion of iPSCs and embryonic stem cells (ESCs). Various laminin combinations exert distinct functions in different tissues, including involvement in basement membrane construction and regulation of cell adhesion, proliferation, migration, and differentiation. Currently, at least 16 different laminin isoform combinations are known, each playing essential roles in cell growth and development.
The remarkable efficacy of laminin in supporting iPSC culture stems from its capacity to bind with various cell surface receptors, particularly integrin receptors. Integrin α6β1 is highly expressed on the surface of iPSCs and ESCs, and laminin isoforms such as laminin-511, -332, and -111 exhibit high binding affinity for integrin α6β1. Functional blocking studies have confirmed that disrupting the integrin α6β1-laminin interaction impedes cell adhesion, underscoring the central role of this receptor in mediating laminin-dependent cell adhesion.
To meet the growing demand for consistent, high-quality laminin matrices for stem cell research, we offer an array of recombinant laminin proteins. These include GMP-grade and premium-grade Laminin 521, premium-grade Laminin 511, and others specifically tailored for iPSC culture applications. These products undergo rigorous validation to ensure dependable performance in supporting iPSC adhesion, proliferation, and maintenance of pluripotency. They are also designed to meet safety and scalability requirements for clinical and research applications.
GMP Laminin 521 Product Data
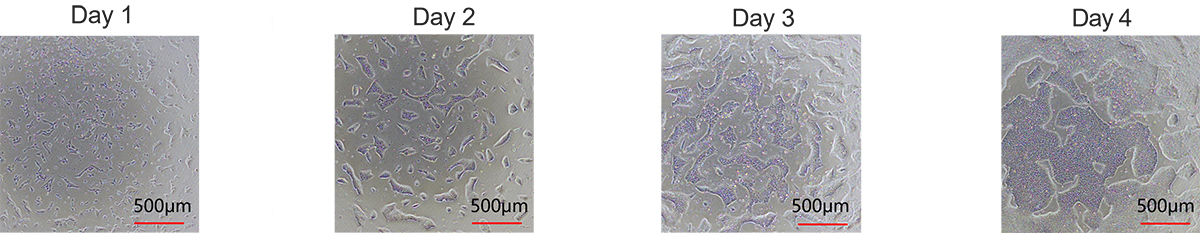
Figure 1. Laminin 521 (GMP-LA5H24) effectively maintains the expansion of human iPSCs.
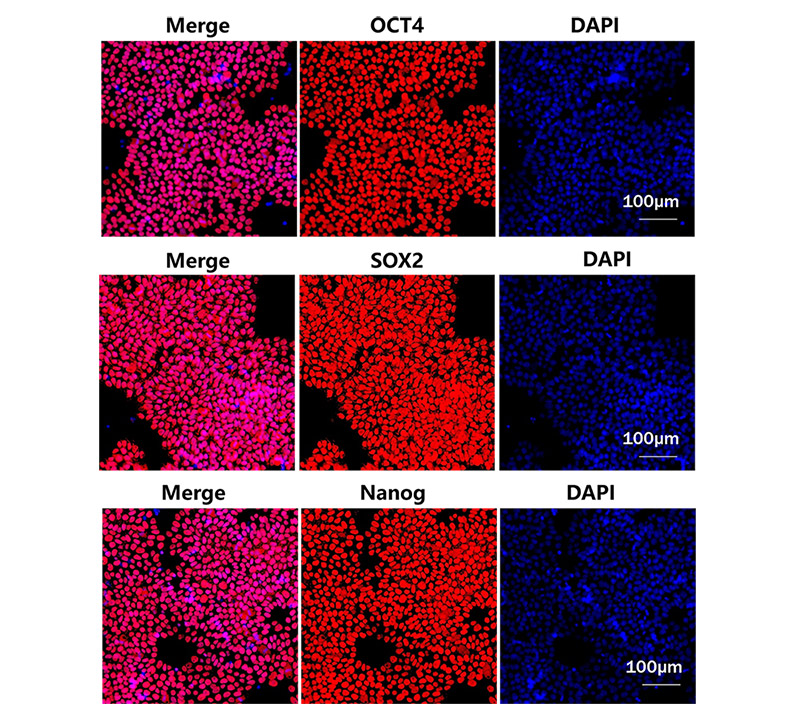
Figure 2. Laminin 521 (GMP-LA5H24) could maintain the stemness of iPSC after several passages.
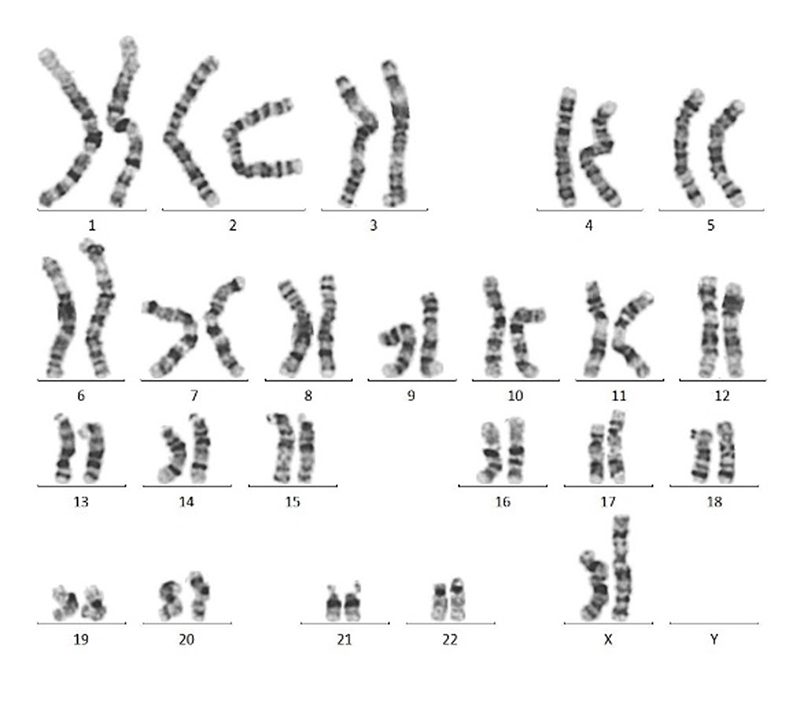
Figure 3. Normal karyotype (46, XX) was found in hiPSCs with Laminin 521(GMP-LA5H24) coating after 10 passages.
This web search service is supported by Google Inc.







